Diffusion-weighted breast MRI: Clinical applications and emerging techniques
- PMID: 27690173
- PMCID: PMC5222835
- DOI: 10.1002/jmri.25479
Diffusion-weighted breast MRI: Clinical applications and emerging techniques
Abstract
Diffusion-weighted MRI (DWI) holds potential to improve the detection and biological characterization of breast cancer. DWI is increasingly being incorporated into breast MRI protocols to address some of the shortcomings of routine clinical breast MRI. Potential benefits include improved differentiation of benign and malignant breast lesions, assessment and prediction of therapeutic efficacy, and noncontrast detection of breast cancer. The breast presents a unique imaging environment with significant physiologic and inter-subject variations, as well as specific challenges to achieving reliable high quality diffusion-weighted MR images. Technical innovations are helping to overcome many of the image quality issues that have limited widespread use of DWI for breast imaging. Advanced modeling approaches to further characterize tissue perfusion, complexity, and glandular organization may expand knowledge and yield improved diagnostic tools.
Level of evidence: 5 J. Magn. Reson. Imaging 2016 J. Magn. Reson. Imaging 2017;45:337-355.
Keywords: breast DWI artifacts; breast cancer diagnostics; breast diffusion-weighted MRI; breast physiology; diffusion tensor imaging; intravoxel incoherent motion.
© 2016 International Society for Magnetic Resonance in Medicine.
Figures
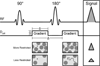
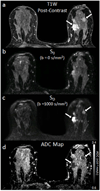


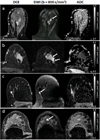

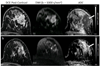
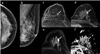
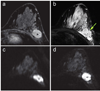
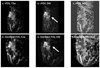

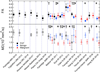
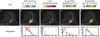
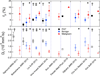
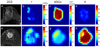
References
-
- Stejskal EOTJ. Spin diffusion measurements:spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;(42):288–292.
-
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. Epub 1986/11/01. PubMed PMID: 3763909. - PubMed
-
- Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. PubMed PMID: 3393671. - PubMed
-
- Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16(2):172–178. PubMed PMID: 12203765. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

