Factors and pathways involved in capacitation: how are they regulated?
- PMID: 27690295
- PMCID: PMC5356907
- DOI: 10.18632/oncotarget.12274
Factors and pathways involved in capacitation: how are they regulated?
Abstract
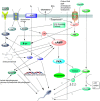
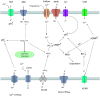
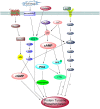
In mammals, fertilization occurs via a comprehensive progression of events. Freshly ejaculated sperm have yet to acquire progressive motility or fertilization ability. They must first undergo a series of biochemical and physiological changes, collectively known as capacitation. Capacitation is a significant prerequisite to fertilization. During the process of capacitation, changes in membrane properties, intracellular ion concentration and the activities of enzymes, together with other protein modifications, induce multiple signaling events and pathways in defined media in vitro or in the female reproductive tract in vivo. These, in turn, stimulate the acrosome reaction and prepare spermatozoa for penetration of the egg zona pellucida prior to fertilization. In the present review, we conclude all mainstream factors and pathways regulate capacitation and highlight their crosstalk. We also summarize the relationship between capacitation and assisted reproductive technology or human disease. In the end, we sum up the open questions and future avenues in this field.
Keywords: cAMP-PKA; calcium ion; capacitation; protein phosphorylation; signaling pathway.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartality of the review.
Figures
Similar articles
-
Kinases, phosphatases and proteases during sperm capacitation.Cell Tissue Res. 2012 Sep;349(3):765-82. doi: 10.1007/s00441-012-1370-3. Epub 2012 Mar 20. Cell Tissue Res. 2012. PMID: 22427115 Review.
-
Signalling Events and Associated Pathways Related to the Mammalian Sperm Capacitation.Reprod Domest Anim. 2015 Oct;50(5):705-11. doi: 10.1111/rda.12541. Epub 2015 Aug 21. Reprod Domest Anim. 2015. PMID: 26294224 Review.
-
Human sperm activation during capacitation and acrosome reaction: role of calcium, protein phosphorylation and lipid remodelling pathways.Front Biosci. 1996 Aug 15;1:d189-205. doi: 10.2741/a125. Front Biosci. 1996. PMID: 9159227 Review.
-
Intracellular events and signaling pathways involved in sperm acquisition of fertilizing capacity and acrosome reaction.Front Biosci. 2000 Nov 1;5:E110-23. doi: 10.2741/baldi. Front Biosci. 2000. PMID: 11056077 Review.
-
Signalling pathways involved in sperm capacitation.Soc Reprod Fertil Suppl. 2007;65:245-59. Soc Reprod Fertil Suppl. 2007. PMID: 17644966 Review.
Cited by
-
Differential sperm proteomic profiles according to pregnancy achievement in intracytoplasmic sperm injection cycles: a pilot study.J Assist Reprod Genet. 2021 Jun;38(6):1507-1521. doi: 10.1007/s10815-021-02098-0. Epub 2021 Apr 9. J Assist Reprod Genet. 2021. PMID: 33835370 Free PMC article.
-
Structure and Function of Ion Channels Regulating Sperm Motility-An Overview.Int J Mol Sci. 2021 Mar 23;22(6):3259. doi: 10.3390/ijms22063259. Int J Mol Sci. 2021. PMID: 33806823 Free PMC article. Review.
-
From TgO/GABA-AT, GABA, and T-263 Mutant to Conception of Toxoplasma.iScience. 2023 Nov 16;27(1):108477. doi: 10.1016/j.isci.2023.108477. eCollection 2024 Jan 19. iScience. 2023. PMID: 38205261 Free PMC article.
-
Lead and calcium crosstalk tempted acrosome damage and hyperpolarization of spermatozoa: signaling and ultra-structural evidences.Biol Res. 2024 Jul 5;57(1):44. doi: 10.1186/s40659-024-00517-x. Biol Res. 2024. PMID: 38965573 Free PMC article.
-
Exogenous Albumin Is Crucial for Pig Sperm to Elicit In Vitro Capacitation Whereas Bicarbonate Only Modulates Its Efficiency.Biology (Basel). 2021 Oct 26;10(11):1105. doi: 10.3390/biology10111105. Biology (Basel). 2021. PMID: 34827098 Free PMC article.
References
-
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. - PubMed
-
- Austin C. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–596. - PubMed
-
- Austin C. The capacitation of the mammalian sperm. Nature. 1952;170:326–332. - PubMed
-
- Imai H, Niwa K, Iritani A. Penetration in vitro of zona-free hamster eggs by ejaculated boar spermatozoa. J Reprod Fertil. 1977;51:495–497. - PubMed
-
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

