On the origin of myeloid-derived suppressor cells
- PMID: 27690299
- PMCID: PMC5356220
- DOI: 10.18632/oncotarget.12278
On the origin of myeloid-derived suppressor cells
Abstract
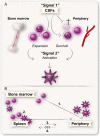
Myeloid-derived suppressor cells (MDSCs) have a strong immunosuppressive character that allows them to regulate immune responses and hinder overt inflammatory responses. In cancer, this leads to tumor immune evasion and disease progression. MDSCs come in at least two forms: monocytic (Mo-MDSCs) and granulocytic (G-MDSCs). The classical definition of MDSCs as immature myeloid cells blocked from differentiating has been challenged by recent studies suggesting that Mo-MDSCs and G-MDSCs may represent monocytes and granulocytes that have acquired immunosuppressive properties. The molecular mechanism behind their generation and their true origins are now widely debated. In this review we discuss the different proposed mechanisms of the generation of both types of MDSCs, with a special focus on human MDSCs in cancer.
Keywords: MDSC origin; emergency myelopoiesis; extramedullary; myelopoiesis; reprogramming.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

