Widespread Shortening of 3' Untranslated Regions and Increased Exon Inclusion Are Evolutionarily Conserved Features of Innate Immune Responses to Infection
- PMID: 27690314
- PMCID: PMC5045211
- DOI: 10.1371/journal.pgen.1006338
Widespread Shortening of 3' Untranslated Regions and Increased Exon Inclusion Are Evolutionarily Conserved Features of Innate Immune Responses to Infection
Abstract
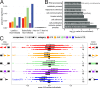
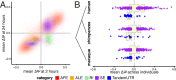
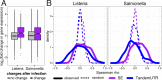
The contribution of pre-mRNA processing mechanisms to the regulation of immune responses remains poorly studied despite emerging examples of their role as regulators of immune defenses. We sought to investigate the role of mRNA processing in the cellular responses of human macrophages to live bacterial infections. Here, we used mRNA sequencing to quantify gene expression and isoform abundances in primary macrophages from 60 individuals, before and after infection with Listeria monocytogenes and Salmonella typhimurium. In response to both bacteria we identified thousands of genes that significantly change isoform usage in response to infection, characterized by an overall increase in isoform diversity after infection. In response to both bacteria, we found global shifts towards (i) the inclusion of cassette exons and (ii) shorter 3' UTRs, with near-universal shifts towards usage of more upstream polyadenylation sites. Using complementary data collected in non-human primates, we show that these features are evolutionarily conserved among primates. Following infection, we identify candidate RNA processing factors whose expression is associated with individual-specific variation in isoform abundance. Finally, by profiling microRNA levels, we show that 3' UTRs with reduced abundance after infection are significantly enriched for target sites for particular miRNAs. These results suggest that the pervasive usage of shorter 3' UTRs is a mechanism for particular genes to evade repression by immune-activated miRNAs. Collectively, our results suggest that dynamic changes in RNA processing may play key roles in the regulation of innate immune responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

