Central Nervous System Injury - A Newly Observed Bystander Effect of Radiation
- PMID: 27690377
- PMCID: PMC5045183
- DOI: 10.1371/journal.pone.0163233
Central Nervous System Injury - A Newly Observed Bystander Effect of Radiation
Abstract
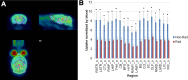
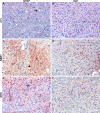
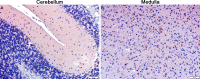
The unintended side effects of cancer treatment are increasing recognized. Among these is a syndrome of long-term neurocognitive dysfunction called cancer/chemotherapy related cognitive impairment. To date, all studies examining the cognitive impact of cancer treatment have emphasized chemotherapy. Radiation-induced bystander effects have been described in cell culture and, to a limited extent, in rodent model systems. The purpose of this study was to examine, for the first time, the impact of non-brain directed radiation therapy on the brain in order to elucidate its potential relationship with cancer/chemotherapy related cognitive impairment. To address this objective, female BALB/c mice received either a single 16 gray fraction of ionizing radiation to the right hind limb or three doses of methotrexate, once per week for three consecutive weeks. Mice were sacrificed either 3 or 30 days post-treatment and brain injury was determined via quantification of activated astrocytes and microglia. To characterize the effects of non-brain directed radiation on brain glucose metabolism, mice were evaluated by fluorodeoxygluocose positron emission tomography. A single fraction of 16 gray radiation resulted in global decreases in brain glucose metabolism, a significant increase in the number of activated astrocytes and microglia, and increased TNF-α expression, all of which lasted up to 30 days post-treatment. This inflammatory response following radiation therapy was statistically indistinguishable from the neuroinflammation observed following methotrexate administration. In conclusion, non-brain directed radiation was sufficient to cause significant brain bystander injury as reflected by multifocal hypometabolism and persistent neuroinflammation. These findings suggest that radiation induces significant brain bystander effects distant from the irradiated cells and tissues. These effects may contribute to the development of cognitive dysfunction in treated human cancer patients and warrant further study.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









Similar articles
-
Cancer treatment induces neuroinflammation and behavioral deficits in mice.Front Behav Neurosci. 2023 Jan 9;16:1067298. doi: 10.3389/fnbeh.2022.1067298. eCollection 2022. Front Behav Neurosci. 2023. PMID: 36699654 Free PMC article.
-
[Radiation-induced bystander effect: the important part of ionizing radiation response. Potential clinical implications].Postepy Hig Med Dosw (Online). 2009 Aug 18;63:377-88. Postepy Hig Med Dosw (Online). 2009. PMID: 19724078 Review. Polish.
-
Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo.Brain Res. 2010 Feb 26;1316:101-11. doi: 10.1016/j.brainres.2009.12.055. Epub 2010 Jan 4. Brain Res. 2010. PMID: 20044983
-
Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis.J Neuroinflammation. 2016 May 31;13(1):127. doi: 10.1186/s12974-016-0592-9. J Neuroinflammation. 2016. PMID: 27245661 Free PMC article.
-
Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects.J Radiat Res. 2007 Mar;48(2):87-95. doi: 10.1269/jrr.06084. Epub 2007 Feb 28. J Radiat Res. 2007. PMID: 17327686 Review.
Cited by
-
Neuroimmunology of Behavioral Comorbidities Associated With Cancer and Cancer Treatments.Front Immunol. 2018 Jun 7;9:1195. doi: 10.3389/fimmu.2018.01195. eCollection 2018. Front Immunol. 2018. PMID: 29930550 Free PMC article. Review.
-
Cancer related cognitive impairment: a downside of cancer treatment.Front Oncol. 2024 Apr 23;14:1387251. doi: 10.3389/fonc.2024.1387251. eCollection 2024. Front Oncol. 2024. PMID: 38715789 Free PMC article. Review.
-
Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults.Neuro Oncol. 2018 Apr 9;20(5):597-607. doi: 10.1093/neuonc/nox195. Neuro Oncol. 2018. PMID: 29045710 Free PMC article. Review.
-
Cancer treatment induces neuroinflammation and behavioral deficits in mice.Front Behav Neurosci. 2023 Jan 9;16:1067298. doi: 10.3389/fnbeh.2022.1067298. eCollection 2022. Front Behav Neurosci. 2023. PMID: 36699654 Free PMC article.
-
Neurotoxicity from Old and New Radiation Treatments for Brain Tumors.Int J Mol Sci. 2023 Jun 26;24(13):10669. doi: 10.3390/ijms241310669. Int J Mol Sci. 2023. PMID: 37445846 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

