Acute and chronic effects of cannabidiol on Δ⁹-tetrahydrocannabinol (Δ⁹-THC)-induced disruption in stop signal task performance
- PMID: 27690502
- PMCID: PMC5119678
- DOI: 10.1037/pha0000081
Acute and chronic effects of cannabidiol on Δ⁹-tetrahydrocannabinol (Δ⁹-THC)-induced disruption in stop signal task performance
Abstract
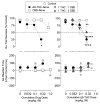
Recent clinical and preclinical research has suggested that cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC) have interactive effects on measures of cognition; however, the nature of these interactions is not yet fully characterized. To address this, we investigated the effects of Δ9-THC and CBD independently and in combination with proposed therapeutic dose ratios of 1:1 and 1:3 Δ9-THC:CBD in adult rhesus monkeys (n = 6) performing a stop signal task (SST). Additionally, the development of tolerance to the effects of Δ9-THC on SST performance was evaluated by determining the effects of acutely administered Δ9-THC (0.1-3.2 mg/kg), during a 24-day chronic Δ9-THC treatment period with Δ9-THC alone or in combination with CBD. Results indicate that Δ9-THC (0.032-0.32 mg/kg) dose-dependently decreased go success but did not alter go reaction time (RT) or stop signal RT (SSRT); CBD (0.1-1.0 mg/kg) was without effect on all measures and, when coadministered in a 1:1 dose ratio, did not exacerbate or attenuate the effects of Δ9-THC. When coadministered in a 1:3 dose ratio, CBD (1.0 mg/kg) attenuated the disruptive effects of 0.32 mg/kg Δ9-THC but did not alter the effects of other Δ9-THC doses. Increases in ED50 values for the effects of Δ9-THC on SST performance were apparent during chronic Δ9-THC treatment, with little evidence for modification of changes in sensitivity by CBD. These results indicate that CBD, when combined with Δ9-THC in clinically available dose ratios, does not exacerbate and, under restricted conditions may even attenuate, Δ9-THC's behavioral effects. (PsycINFO Database Record
(c) 2016 APA, all rights reserved).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice.Int J Neuropsychopharmacol. 2010 Aug;13(7):861-76. doi: 10.1017/S1461145709990605. Epub 2009 Sep 29. Int J Neuropsychopharmacol. 2010. PMID: 19785914
-
Assessment of Orally Administered Δ9-Tetrahydrocannabinol When Coadministered With Cannabidiol on Δ9-Tetrahydrocannabinol Pharmacokinetics and Pharmacodynamics in Healthy Adults: A Randomized Clinical Trial.JAMA Netw Open. 2023 Feb 1;6(2):e2254752. doi: 10.1001/jamanetworkopen.2022.54752. JAMA Netw Open. 2023. PMID: 36780161 Free PMC article. Clinical Trial.
-
A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects.Eur Arch Psychiatry Clin Neurosci. 2019 Feb;269(1):17-35. doi: 10.1007/s00406-019-00978-2. Epub 2019 Jan 19. Eur Arch Psychiatry Clin Neurosci. 2019. PMID: 30661105 Clinical Trial.
-
How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review.Neurosci Biobehav Rev. 2019 Dec;107:696-712. doi: 10.1016/j.neubiorev.2019.09.036. Epub 2019 Sep 30. Neurosci Biobehav Rev. 2019. PMID: 31580839
-
Does Cannabis Composition Matter? Differential Effects of Delta-9-tetrahydrocannabinol and Cannabidiol on Human Cognition.Curr Addict Rep. 2017;4(2):62-74. doi: 10.1007/s40429-017-0142-2. Epub 2017 Apr 29. Curr Addict Rep. 2017. PMID: 28580227 Free PMC article. Review.
Cited by
-
Neuronal and Astrocytic Morphological Alterations Driven by Prolonged Exposure with Δ9-Tetrahydrocannabinol but Not Cannabidiol.Toxics. 2022 Jan 21;10(2):48. doi: 10.3390/toxics10020048. Toxics. 2022. PMID: 35202235 Free PMC article.
-
Cannabidiol as a Promising Strategy to Treat and Prevent Movement Disorders?Front Pharmacol. 2018 May 11;9:482. doi: 10.3389/fphar.2018.00482. eCollection 2018. Front Pharmacol. 2018. PMID: 29867488 Free PMC article. Review.
-
Delta-9-Tetrahydrocannabinol, Cannabidiol, and Acute Psychotomimetic States: A Balancing Act of the Principal Phyto-Cannabinoids on Human Brain and Behavior.Cannabis Cannabinoid Res. 2023 Oct;8(5):846-856. doi: 10.1089/can.2021.0166. Epub 2022 Mar 18. Cannabis Cannabinoid Res. 2023. PMID: 35319274 Free PMC article. Clinical Trial.
-
Astrogliosis Occurs Selectively in Amygdala of Adolescent Primate and Rodent Following Daily Δ9-Tetrahydrocannabinol, Prevented by Cannabidiol Co-Treatment.Biol Psychiatry Glob Open Sci. 2025 Mar 29;5(4):100496. doi: 10.1016/j.bpsgos.2025.100496. eCollection 2025 Jul. Biol Psychiatry Glob Open Sci. 2025. PMID: 40487783 Free PMC article.
-
Cannabidiol modulation of antinociceptive tolerance to Δ9-tetrahydrocannabinol.Psychopharmacology (Berl). 2018 Nov;235(11):3289-3302. doi: 10.1007/s00213-018-5036-z. Epub 2018 Sep 20. Psychopharmacology (Berl). 2018. PMID: 30238130 Free PMC article.
References
-
- Babalonis S, Lofwall MR, Nuzzo PA, Elayi C, Malcolm RJ, Haney M, Walsh SL. Examination of the behavioral effects of oral cannabidiol alone and in combination with smoked marijuana. Drug and Alcohol Dependence. 2015 http://doi.org/10.1016/j.drugalcdep.2015.07.953. - DOI
-
- Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology. 2001;134(4):845–852. http://doi.org/10.1038/sj.bjp.0704327. - DOI - PMC - PubMed
-
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural Basis of Δ-9-Tetrahydrocannabinol and Cannabidiol: Effects During Response Inhibition. Biological Psychiatry. 2008;64(11):966–973. http://doi.org/10.1016/j.biopsych.2008.05.011. - DOI - PubMed
-
- Brady KT, Balster RL. The effects of Δ9-tetrahydrocannabinol alone and in combination with cannabidiol on fixed-interval performance in rhesus monkeys. Psychopharmacology. 1980;72(1):21–26. http://doi.org/10.1007/BF00433803. - DOI - PubMed
-
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of Cannabis on Neurocognitive Functioning: Recent Advances, Neurodevelopmental Influences, and Sex Differences. Neuropsychology Review. 2013;23(2):117–137. http//:doi.org/10.1007/s11065-012-9222-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

