Immunogenicity of AS03-adjuvanted and non-adjuvanted trivalent inactivated influenza vaccines in elderly adults: A Phase 3, randomized trial and post-hoc correlate of protection analysis
- PMID: 27690762
- PMCID: PMC5215410
- DOI: 10.1080/21645515.2016.1219809
Immunogenicity of AS03-adjuvanted and non-adjuvanted trivalent inactivated influenza vaccines in elderly adults: A Phase 3, randomized trial and post-hoc correlate of protection analysis
Abstract
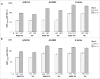
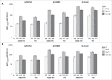
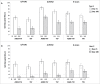
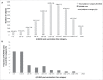
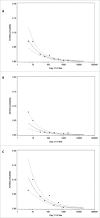
In this study we describe the immunogenicity results from a subset of older people (N = 5187) who participated in a Phase 3 randomized, observer-blinded trial of AS03-TIV versus TIV (Fluarix™) (ClinicalTrials.gov, NCT00753272). Participants received one dose of AS03-TIV or TIV in each study year and antibody titers against the vaccine strains were assessed using hemagglutination-inhibition (HI) assay at 21 d and 180 d post-vaccination in each vaccine group in the 2008/09 (Year 1) and 2009/10 (Year 2) influenza seasons. Manufacturing consistency of 3 lots of AS03-TIV for HI antibody responses in Year 1 was a co-primary objective. In a post-hoc analysis, a statistical regression model included 4830 subjects in whom immunogenicity and laboratory-confirmed attack rate data were available; the analysis was performed to assess HI antibody titers against A/H3N2 as a correlate of protection for laboratory-confirmed A/H3N2 influenza. AS03-TIV and TIV elicited strong HI antibody responses against each vaccine strain 21 d post-vaccination in both years. The manufacturing consistency of 3 lots of AS03-TIV was demonstrated. In both years and each vaccine group, HI antibody responses were lower for A/H1N1 than the other vaccine strains. Day 180 seroconversion rates (proportion with ≥4-fold increase in titer compared with pre-vaccination titer) in Year 1 in the AS03-TIV and TIV groups, respectively, were 87.7% and 74.1% for A/H3N2, 69.7% and 59.6% for influenza B, and 58.3% and 47.4% for A/H1N1. The post-hoc statistical model based on A/H3N2 attack rates and HI antibody titers estimated that a 4-fold increase in post-vaccination titers against A/H3N2 was associated with a 2-fold decrease in the odds of A/H3N2 infection.
Keywords: AS03; correlates of protection; immunogenicity; older; seasonal influenza; vaccine.
Figures

References
-
- McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine 2005; 23(Suppl 1):S10-25 - PubMed
-
- McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? J Infect Dis 2008; 198:632-4; PMID:18652548; http://dx.doi.org/ 10.1086/590435 - DOI - PubMed
-
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet (London, England) 2005; 366:1165-74; PMID:16198765; http://dx.doi.org/ 10.1016/S0140-6736(05)67339-4 - DOI - PubMed
-
- Arakane R, Annaka R, Takahama A, Ishida K, Yoshiike M, Nakayama T, Takeshita F. Superior immunogenicity profile of the new intradermal influenza vaccine compared to the standard subcutaneous vaccine in subjects 65 years and older: A randomized controlled phase III study. Vaccine 2015; 33:6650-8; PMID:26519549; http://dx.doi.org/ 10.1016/j.vaccine.2015.10.088 - DOI - PubMed
-
- DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009-2010 season. Vaccine 2013; 31:861-6; PMID:23261045; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.013 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
