The Intracellular Cholesterol Landscape: Dynamic Integrator of the Immune Response
- PMID: 27692616
- PMCID: PMC5135597
- DOI: 10.1016/j.it.2016.09.001
The Intracellular Cholesterol Landscape: Dynamic Integrator of the Immune Response
Abstract
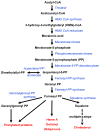
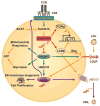
Cholesterol has typically been considered an exogenous, disease-related factor in immunity; however, recent literature suggests that a paradigm shift is in order. Sterols are now recognized to ligate several immune receptors. Altered flux through the mevalonic acid synthesis pathway also appears to be a required event in the antiviral interferon (IFN) response of macrophages and in the activation, proliferation, and differentiation of T cells. In this review, evidence is discussed that suggests an intrinsic, 'professional' role for sterols and oxysterols in macrophage and T-cell immunity. Host defense may have been the original selection pressure behind the development of mechanisms for intracellular cholesterol homeostasis. Functional coupling between sterol metabolism and immunity has fundamental implications for health and disease.
Published by Elsevier Ltd.
Figures

References
-
- Howe V, et al. Cholesterol homeostasis: How do cells sense sterol excess? Chemistry and physics of lipids. 2016 pii: S0009-3084(0016)30033-30030. - PubMed
-
- Lange Y, Steck TL. Active membrane cholesterol as a physiological effector. Chemistry and physics of lipids. 2016 pii: S0009-3084(0016)30015-30019. - PubMed
-
- Kulig W, et al. Cholesterol oxidation products and their biological importance. Chemistry and physics of lipids. 2016 pii: S0009-3084(0016)30034-30032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

