Imbalanced Activity in the Orbitofrontal Cortex and Nucleus Accumbens Impairs Behavioral Inhibition
- PMID: 27693139
- PMCID: PMC5117684
- DOI: 10.1016/j.cub.2016.08.034
Imbalanced Activity in the Orbitofrontal Cortex and Nucleus Accumbens Impairs Behavioral Inhibition
Abstract
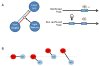
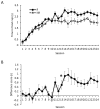
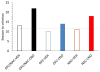
Contemporary models of behavioral regulation maintain that balanced activity between cognitive control areas (prefrontal cortex, PFC) and subcortical reward-related regions (nucleus accumbens, NAC) mediates the selection of appropriate behavioral responses, whereas imbalanced activity (PFC < NAC) results in maladaptive behavior [1-6]. Imbalance can arise from reduced engagement of PFC (via fatigue or stress [7]) or from excessive activity in NAC [8]. Additionally, a concept far less researched is that an imbalance can result from simultaneously low PFC activity and high NAC activity. This occurs during adolescence, when the maturation of PFC lags behind that of NAC and NAC is more functionally active compared to adulthood or pre-adolescence [2, 5, 9, 10]. Accordingly, activity is disproportionately higher in NAC than in PFC, which may contribute to impulsivity and risk-taking exhibited by adolescents [5, 6, 10-12]. Despite having explanatory value, support for this notion has been solely correlational. Here, we causally tested this using chemogenetics to simultaneously decrease neural activity in the orbitofrontal cortex (OFC) and increase activity in NAC in adult rats, mimicking the imbalance during adolescence. We tested the effects on negative occasion setting, an important yet understudied form of inhibitory learning that may be particularly relevant during adolescence. Rats with combined manipulation of OFC and NAC were impaired in learning to use environmental cues to withhold a response, an effect that was greater than that of either manipulation alone. These findings provide direct evidence that simultaneous underactivity in OFC and overactivity in NAC can negatively impact behavioral control and provide insight into the neural systems that underlie inhibitory learning.
Keywords: DREADDs; adolescence; associative learning; chemogenetics; conditioning; learning; negative occasion setting; prefrontal cortex.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Systems Neuroscience: The Balancing Act of Behavioral Regulation.Curr Biol. 2016 Oct 24;26(20):R925-R926. doi: 10.1016/j.cub.2016.08.065. Curr Biol. 2016. PMID: 27780061
-
Resisting the Urge to Act: DREADDs Modifying Habits.Trends Neurosci. 2017 Feb;40(2):61-62. doi: 10.1016/j.tins.2016.12.002. Epub 2017 Jan 16. Trends Neurosci. 2017. PMID: 28104285 Free PMC article.
References
-
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual systems perspective. Perspect Psychol Sci. 2009;4:162–176. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

