The impact of cerebrovascular aging on vascular cognitive impairment and dementia
- PMID: 27693240
- PMCID: PMC5250548
- DOI: 10.1016/j.arr.2016.09.007
The impact of cerebrovascular aging on vascular cognitive impairment and dementia
Abstract
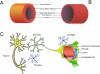
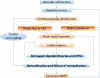
As human life expectancy rises, the aged population will increase. Aging is accompanied by changes in tissue structure, often resulting in functional decline. For example, aging within blood vessels contributes to a decrease in blood flow to important organs, potentially leading to organ atrophy and loss of function. In the central nervous system, cerebral vascular aging can lead to loss of the integrity of the blood-brain barrier, eventually resulting in cognitive and sensorimotor decline. One of the major of types of cognitive dysfunction due to chronic cerebral hypoperfusion is vascular cognitive impairment and dementia (VCID). In spite of recent progress in clinical and experimental VCID research, our understanding of vascular contributions to the pathogenesis of VCID is still very limited. In this review, we summarize recent findings on VCID, with a focus on vascular age-related pathologies and their contribution to the development of this condition.
Keywords: Blood-brain barrier; Hypoperfusion; Vascular aging; White matter.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures

References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiology of disease. 2010;37:13–25. - PubMed
-
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD (P) H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285:H1015–H1022. - PubMed
-
- Ahmad S. Angiotensin receptor antagonists delay nitric oxide-deficient stroke in stroke-prone rats. Eur J Pharmacol. 1997;333:39–45. - PubMed
-
- Ainslie PN. Have a safe night: intimate protection against cerebral hyperperfusion during REM sleep. Journal of Applied Physiology. 2009;106:1031–1033. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

