Tissue Immunometabolism: Development, Physiology, and Pathobiology
- PMID: 27693378
- PMCID: PMC5226870
- DOI: 10.1016/j.cmet.2016.08.016
Tissue Immunometabolism: Development, Physiology, and Pathobiology
Abstract
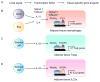
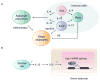
Evolution of metazoans resulted in the specialization of cellular and tissue function. This was accomplished by division of labor, which allowed tissue parenchymal cells to prioritize their core functions while ancillary functions were delegated to tissue accessory cells, such as immune, stromal, and endothelial cells. In metabolic organs, the accessory cells communicate with their clients, the tissue parenchymal cells, to optimize cellular processes, allowing organisms to adapt to changes in their environment. Here, we discuss tissue immunometabolism from this vantage point and use examples from adipose tissues (white, beige, and brown) and liver to outline the general principles by which accessory cells support metabolic homeostasis in parenchymal cells. A corollary of this model is that disruption of communication between client and accessory cells might predispose metabolic organs to the development of disease.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Agarwal P, Khan SR, Verma SC, Beg M, Singh K, Mitra K, Gaikwad AN, Akhtar MS, Krishnan MY. Mycobacterium tuberculosis persistence in various adipose depots of infected mice and the effect of anti-tubercular therapy. Microbes Infect. 2014;16:571–580. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

