Nucleobases Undergo Dynamic Rearrangements during RNA Tertiary Folding
- PMID: 27693721
- PMCID: PMC5085838
- DOI: 10.1016/j.jmb.2016.09.015
Nucleobases Undergo Dynamic Rearrangements during RNA Tertiary Folding
Abstract
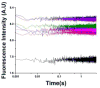
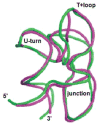
The tertiary structure of the GTPase center (GAC) of 23S ribosomal RNA (rRNA) as seen in cocrystals is extremely compact. It is stabilized by long-range hydrogen bonds and nucleobase stacking and by a triloop that forms within its three-way junction. Its folding pathway from secondary structure to tertiary structure has not been previously observed, but it was shown to require Mg2+ ions in equilibrium experiments. The fluorescent nucleotide 2-aminopurine was substituted at selected sites within the 60-nt GAC. Fluorescence intensity changes upon addition of MgCl2 were monitored over a time-course from 1ms to 100s as the RNA folds. The folding pathway is revealed here to be hierarchical through several intermediates. Observation of the nucleobases during folding provides a new perspective on the process and the pathway, revealing the dynamics of nucleobase conformational exchange during the folding transitions.
Keywords: 2-aminopurine fluorescence; GTPase center RNA; RNA folding kinetics; stopped-flow fluorescence.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
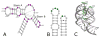



Similar articles
-
Divalent ions tune the kinetics of a bacterial GTPase center rRNA folding transition from secondary to tertiary structure.RNA. 2018 Dec;24(12):1828-1838. doi: 10.1261/rna.068361.118. Epub 2018 Sep 25. RNA. 2018. PMID: 30254137 Free PMC article.
-
Formation of Tertiary Interactions during rRNA GTPase Center Folding.J Mol Biol. 2015 Aug 28;427(17):2799-815. doi: 10.1016/j.jmb.2015.07.013. Epub 2015 Jul 22. J Mol Biol. 2015. PMID: 26210661 Free PMC article.
-
2-Aminopurine Fluorescence as a Probe of Local RNA Structure and Dynamics and Global Folding.Methods Enzymol. 2015;558:99-124. doi: 10.1016/bs.mie.2015.01.006. Epub 2015 Mar 3. Methods Enzymol. 2015. PMID: 26068739
-
Mechanism of ribosome assisted protein folding: a new insight into rRNA functions.Biochem Biophys Res Commun. 2009 Jun 26;384(2):137-40. doi: 10.1016/j.bbrc.2009.04.106. Epub 2009 May 3. Biochem Biophys Res Commun. 2009. PMID: 19401192 Review.
-
Metal ions and RNA folding: a highly charged topic with a dynamic future.Curr Opin Chem Biol. 2005 Apr;9(2):104-9. doi: 10.1016/j.cbpa.2005.02.004. Curr Opin Chem Biol. 2005. PMID: 15811793 Review.
Cited by
-
Translation Fidelity and Respiration Deficits in CLPP-Deficient Tissues: Mechanistic Insights from Mitochondrial Complexome Profiling.Int J Mol Sci. 2023 Dec 15;24(24):17503. doi: 10.3390/ijms242417503. Int J Mol Sci. 2023. PMID: 38139332 Free PMC article.
-
Revealing the distinct folding phases of an RNA three-helix junction.Nucleic Acids Res. 2018 Aug 21;46(14):7354-7365. doi: 10.1093/nar/gky363. Nucleic Acids Res. 2018. PMID: 29762712 Free PMC article.
-
Ribosomal Protein L11 Selectively Stabilizes a Tertiary Structure of the GTPase Center rRNA Domain.J Mol Biol. 2020 Feb 14;432(4):991-1007. doi: 10.1016/j.jmb.2019.12.010. Epub 2019 Dec 24. J Mol Biol. 2020. PMID: 31874150 Free PMC article.
-
Divalent ions tune the kinetics of a bacterial GTPase center rRNA folding transition from secondary to tertiary structure.RNA. 2018 Dec;24(12):1828-1838. doi: 10.1261/rna.068361.118. Epub 2018 Sep 25. RNA. 2018. PMID: 30254137 Free PMC article.
References
-
- Conn GL, Gittis AG, Lattman EE, Misra VK, Draper DE. A compact RNA tertiary structure contains a buried backbone-K+ complex. J Mol Biol. 2002;318:963–73. - PubMed
-
- Wimberly BT, Guymon R, McCutcheon JP, White SW, Ramakrishnan V. A detailed view of a ribosomal active site: the structure of the L11-RNA complex. Cell. 1999;97:491–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

