Hypoxia-inducible factor-2α plays a role in mediating oesophagitis in GORD
- PMID: 27694141
- PMCID: PMC5464991
- DOI: 10.1136/gutjnl-2016-312595
Hypoxia-inducible factor-2α plays a role in mediating oesophagitis in GORD
Abstract
Objective: In an earlier study wherein we induced acute reflux by interrupting proton pump inhibitor (PPI) therapy in patients with reflux oesophagitis (RO) healed by PPIs, we refuted the traditional concept that RO develops as an acid burn. The present study explored our alternative hypothesis that RO results from reflux-stimulated production of pro-inflammatory molecules mediated by hypoxia-inducible factors (HIFs).
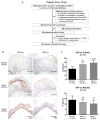
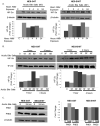
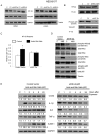
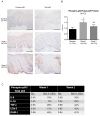
Design: Using oesophageal biopsies taken from patients in our earlier study at baseline and at 1 and 2 weeks off PPIs, we immunostained for HIF-1α, HIF-2α and phospho-p65, and measured pro-inflammatory molecule mRNAs. We exposed human oesophageal squamous cell lines to acidic bile salts, and evaluated effects on HIF activation, p65 function, pro-inflammatory molecule production and immune cell migration.
Results: In patient biopsies, increased immunostaining for HIF-2α and phospho-p65, and increased pro-inflammatory molecule mRNA levels were seen when RO redeveloped 1 or 2 weeks after stopping PPIs. In oesophageal cells, exposure to acidic bile salts increased intracellular reactive oxygen species, which decreased prolyl hydroxylase function and stabilised HIF-2α, causing a p65-dependent increase in pro-inflammatory molecules; conditioned media from these cells increased T cell migration rates. HIF-2α inhibition by small hairpin RNA or selective small molecule antagonist blocked the increases in pro-inflammatory molecule expression and T cell migration induced by acidic bile salts.
Conclusions: In patients developing RO, increases in oesophageal HIF-2α correlate with increased pro-inflammatory molecule expression. In oesophageal epithelial cells, acidic bile salts stabilise HIF-2α, which mediates expression of pro-inflammatory molecules. HIF-2α appears to have a role in RO pathogenesis.
Trial registration number: NCT01733810; Results.
Keywords: CELL SIGNALLING; CYTOKINES; INFLAMMATORY DISEASES; OESOPHAGEAL DISEASE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Figures






References
-
- Winkelstein A. Peptic esophagitis: a new clinical entity. Jama. 1935;104:906–909.
-
- Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58:163–74. - PubMed
-
- Frierson HF., Jr Histology in the diagnosis of reflux esophagitis. Gastroenterol Clin North Am. 1990;19:631–44. - PubMed
-
- Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–84. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
