Novel Role for Matrix Metalloproteinase 9 in Modulation of Cholesterol Metabolism
- PMID: 27694328
- PMCID: PMC5121519
- DOI: 10.1161/JAHA.116.004228
Novel Role for Matrix Metalloproteinase 9 in Modulation of Cholesterol Metabolism
Abstract
Background: The development of atherosclerosis is strongly linked to disorders of cholesterol metabolism. Matrix metalloproteinases (MMPs) are dysregulated in patients and animal models with atherosclerosis. Whether systemic MMP activity influences cholesterol metabolism is unknown.
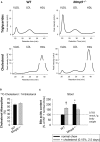
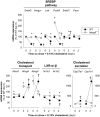
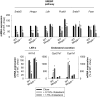
Methods and results: We examined MMP-9-deficient (Mmp9-/-) mice and found them to have abnormal lipid gene transcriptional responses to dietary cholesterol supplementation. As opposed to Mmp9+/+ (wild-type) mice, Mmp9-/- mice failed to decrease the hepatic expression of sterol regulatory element binding protein 2 pathway genes, which control hepatic cholesterol biosynthesis and uptake. Furthermore, Mmp9-/- mice failed to increase the expression of genes encoding the rate-limiting enzymes in biliary cholesterol excretion (eg, Cyp7a and Cyp27a). In contrast, MMP-9 deficiency did not impair intestinal cholesterol absorption, as shown by the 14C-cholesterol and 3H-sitostanol absorption assay. Similar to our earlier study on Mmp2-/- mice, we observed that Mmp9-/- mice had elevated plasma secreted phospholipase A2 activity. Pharmacological inhibition of systemic circulating secreted phospholipase A2 activity (with varespladib) partially normalized the hepatic transcriptional responses to dietary cholesterol in Mmp9-/- mice. Functional studies with mice deficient in other MMPs suggested an important role for the MMP system, as a whole, in modulation of cholesterol metabolism.
Conclusions: Our results show that MMP-9 modulates cholesterol metabolism, at least in part, through a novel MMP-9-plasma secreted phospholipase A2 axis that affects the hepatic transcriptional responses to dietary cholesterol. Furthermore, the data suggest that dysregulation of the MMP system can result in metabolic disorder, which could lead to atherosclerosis and coronary heart disease.
Keywords: atherosclerosis; cholesterol; lipid metabolism; liver; matrix metalloproteinase; plasma phospholipase A2.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures



Similar articles
-
Identification of a Novel Heart-Liver Axis: Matrix Metalloproteinase-2 Negatively Regulates Cardiac Secreted Phospholipase A2 to Modulate Lipid Metabolism and Inflammation in the Liver.J Am Heart Assoc. 2015 Nov 13;4(11):e002553. doi: 10.1161/JAHA.115.002553. J Am Heart Assoc. 2015. PMID: 26567374 Free PMC article.
-
Downregulation of Cyp7a1 by Cholic Acid and Chenodeoxycholic Acid in Cyp27a1/ApoE Double Knockout Mice: Differential Cardiovascular Outcome.Front Endocrinol (Lausanne). 2020 Oct 28;11:586980. doi: 10.3389/fendo.2020.586980. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193099 Free PMC article.
-
Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever.J Am Heart Assoc. 2015 Mar 27;4(4):e001868. doi: 10.1161/JAHA.115.001868. J Am Heart Assoc. 2015. PMID: 25820137 Free PMC article.
-
Phospholipase A2s: developing drug targets for atherosclerosis.Atherosclerosis. 2010 Oct;212(2):357-66. doi: 10.1016/j.atherosclerosis.2010.03.011. Epub 2010 Mar 16. Atherosclerosis. 2010. PMID: 20363471 Review.
-
Modulation of Systemic Metabolism by MMP-2: From MMP-2 Deficiency in Mice to MMP-2 Deficiency in Patients.Compr Physiol. 2016 Sep 15;6(4):1935-1949. doi: 10.1002/cphy.c160010. Compr Physiol. 2016. PMID: 27783864 Review.
Cited by
-
MMP-2/9 inhibition modulates sharp wave abundance, inhibitory proteoglycan sulfation, and fear memory in juvenile zebrafish: relevance to affective disorders.Mol Psychiatry. 2025 Sep;30(9):4258-4273. doi: 10.1038/s41380-025-03007-y. Epub 2025 May 2. Mol Psychiatry. 2025. PMID: 40316676
-
Increased MMP-9 levels with strain-dependent stress resilience and tunnel handling in mice.Behav Brain Res. 2021 Jun 25;408:113288. doi: 10.1016/j.bbr.2021.113288. Epub 2021 Apr 6. Behav Brain Res. 2021. PMID: 33836170 Free PMC article.
-
Impact of Epigenetics, Diet, and Nutrition-Related Pathologies on Wound Healing.Int J Mol Sci. 2024 Sep 28;25(19):10474. doi: 10.3390/ijms251910474. Int J Mol Sci. 2024. PMID: 39408801 Free PMC article. Review.
-
Evaluation of MMP-9, IL-6, TNF-α levels and peripheral blood mononuclear cells genes expression of MMP-9 and TIMP-1 in Iranian patients with coronary artery disease.J Cardiovasc Thorac Res. 2023;15(4):223-230. doi: 10.34172/jcvtr.2023.31844. Epub 2023 Dec 30. J Cardiovasc Thorac Res. 2023. PMID: 38357561 Free PMC article.
-
Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability.Int J Mol Sci. 2020 May 31;21(11):3946. doi: 10.3390/ijms21113946. Int J Mol Sci. 2020. PMID: 32486345 Free PMC article. Review.
References
-
- Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. - PubMed
-
- Hernandez‐Anzaldo S, Berry E, Brglez V, Leung D, Yun TJ, Lee JS, Filep JG, Kassiri Z, Cheong C, Lambeau G, Lehner R, Fernandez‐Patron C. Identification of a novel heart‐liver axis: matrix metalloproteinase‐2 negatively regulates cardiac secreted phospholipase A2 to modulate lipid metabolism and inflammation in the liver. J Am Heart Assoc. 2015;4:e002553 doi: 10.1161/JAHA.115.002553. - DOI - PMC - PubMed
-
- Berry E, Hernandez‐Anzaldo S, Ghomashchi F, Lehner R, Murakami M, Gelb MH, Kassiri Z, Wang X, Fernandez‐Patron C. Matrix metalloproteinase‐2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J Am Heart Assoc. 2015;4:e001868 doi: 10.1161/JAHA.115.001868. - DOI - PMC - PubMed
-
- Silvello D, Narvaes LB, Albuquerque LC, Forgiarini LF, Meurer L, Martinelli NC, Andrades ME, Clausell N, dos Santos KG, Rohde LE. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: higher MMP‐9 levels are associated with plaque vulnerability. Biomarkers. 2014;19:49–55. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

