AT-RvD1 combined with DEX is highly effective in treating TNF-α-mediated disruption of the salivary gland epithelium
- PMID: 27694530
- PMCID: PMC5064142
- DOI: 10.14814/phy2.12990
AT-RvD1 combined with DEX is highly effective in treating TNF-α-mediated disruption of the salivary gland epithelium
Abstract
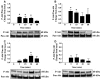
Sjögren's syndrome (SS) is an autoimmune disorder characterized by chronic inflammation and destruction of salivary and lacrimal glands leading to dry mouth and dry eyes, respectively. Currently, the etiology of SS is unknown and the current therapies have no permanent benefit; therefore, new approaches are necessary to effectively treat this condition. Resolvins are highly potent endogenous lipid mediators that are synthesized during the resolution of inflammation to restore tissue homeostasis. Previous studies indicate that the resolvin family member, RvD1, binds to the ALX/FPR2 receptor to block inflammatory signals caused by tumor necrosis factor-alpha (TNF-α) in the salivary epithelium. More recently, the corticosteroid, dexamethasone (DEX), was shown to be effective in reducing salivary gland inflammation. However, DEX, as with other corticosteroids, elicits adverse secondary effects that could be ameliorated when used in smaller doses. Therefore, we investigated whether the more stable aspirin-triggered (AT) epimer, AT-RvD1, combined with reduced doses of DEX is effective in treating TNF-α-mediated disruption of polarized rat parotid gland (Par-C10) epithelial cell clusters. Our results indicate that AT-RvD1 and DEX individually reduced TNF-α-mediated alteration in the salivary epithelium (i.e, maintained cell cluster formation, increased lumen size, reduced apoptosis, and preserved cell survival signaling responses) as compared to untreated cells. Furthermore, AT-RvD1 combined with a reduced dose of DEX produced stronger responses (i.e., robust salivary cell cluster formation, larger lumen sizes, further reduced apoptosis, and sustained survival signaling responses) as compared to those observed with individual treatments. These studies demonstrate that AT-RvD1 combined with DEX is highly effective in treating TNF-α-mediated disruption of salivary gland epithelium.
Keywords: ALX/FPR2; AT‐RvD1; RvD1; Sjögren's syndrome; salivary glands.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures



Similar articles
-
ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands.Am J Physiol Cell Physiol. 2014 Jan 15;306(2):C178-85. doi: 10.1152/ajpcell.00284.2013. Epub 2013 Nov 20. Am J Physiol Cell Physiol. 2014. PMID: 24259417 Free PMC article.
-
Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation.Am J Physiol Cell Physiol. 2012 May 1;302(9):C1331-45. doi: 10.1152/ajpcell.00207.2011. Epub 2012 Jan 11. Am J Physiol Cell Physiol. 2012. PMID: 22237406 Free PMC article.
-
AT-RvD1 Promotes Resolution of Inflammation in NOD/ShiLtJ mice.Sci Rep. 2017 Mar 31;7:45525. doi: 10.1038/srep45525. Sci Rep. 2017. PMID: 28361884 Free PMC article.
-
Immunohistopathology of Sjögren's syndrome.Autoimmun Rev. 2006 Nov;6(1):16-20. doi: 10.1016/j.autrev.2006.03.003. Epub 2006 Apr 19. Autoimmun Rev. 2006. PMID: 17110311 Review.
-
Current experimental methods to investigate the impact of specialized pro-resolving lipid mediators on Sjögren's syndrome.Front Immunol. 2023 Jan 12;13:1094278. doi: 10.3389/fimmu.2022.1094278. eCollection 2022. Front Immunol. 2023. PMID: 36713415 Free PMC article. Review.
Cited by
-
Role of Resolvins in the Inflammatory Resolution of Neurological Diseases.Front Pharmacol. 2020 May 8;11:612. doi: 10.3389/fphar.2020.00612. eCollection 2020. Front Pharmacol. 2020. PMID: 32457616 Free PMC article. Review.
-
The role of peripheral inflammatory insults in Alzheimer's disease: a review and research roadmap.Mol Neurodegener. 2023 Jun 5;18(1):37. doi: 10.1186/s13024-023-00627-2. Mol Neurodegener. 2023. PMID: 37277738 Free PMC article. Review.
-
Sex Differences in Otolaryngology: Focus on the Emerging Role of Estrogens in Inflammatory and Pro-Resolving Responses.Int J Mol Sci. 2021 Aug 16;22(16):8768. doi: 10.3390/ijms22168768. Int J Mol Sci. 2021. PMID: 34445474 Free PMC article. Review.
-
Predicting Resolvin D1 Pharmacokinetics in Humans with Physiologically-Based Pharmacokinetic Modeling.Clin Transl Sci. 2021 Mar;14(2):683-691. doi: 10.1111/cts.12930. Epub 2020 Nov 30. Clin Transl Sci. 2021. PMID: 33202089 Free PMC article.
-
A combination treatment of low-dose dexamethasone and aspirin-triggered resolvin D1 reduces Sjögren syndrome-like features in a mouse model.JADA Found Sci. 2023;2:100016. doi: 10.1016/j.jfscie.2022.100016. Epub 2022 Nov 17. JADA Found Sci. 2023. PMID: 37622089 Free PMC article.
References
-
- Baker, O. J. , Camden J. M., Redman R. S., Jones J. E., Seye C. I., Erb L., et al. 2008. Proinflammatory cytokines tumor necrosis factor‐α and interferon‐γ alter tight junction structure and function in the rat parotid gland Par‐C10 cell line. Am. J. Physiol. Cell Physiol. 295:C1191–C1201. - PMC - PubMed
-
- Boumba, D. , Skopouli F., and Moutsopoulos H.. 1995. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren's syndrome. Rheumatology 34:326–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

