Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer
- PMID: 27694579
- PMCID: PMC5081658
- DOI: 10.1073/pnas.1614529113
Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer
Abstract
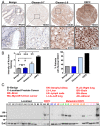
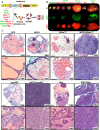
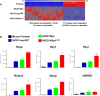
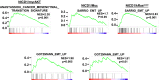
Metastatic castration-resistant prostate cancer (CRPC) is the primary cause of prostate cancer-specific mortality. Defining new mechanisms that can predict recurrence and drive lethal CRPC is critical. Here, we demonstrate that localized high-risk prostate cancer and metastatic CRPC, but not benign prostate tissues or low/intermediate-risk prostate cancer, express high levels of nuclear Notch homolog 1, translocation-associated (Notch1) receptor intracellular domain. Chronic activation of Notch1 synergizes with multiple oncogenic pathways altered in early disease to promote the development of prostate adenocarcinoma. These tumors display features of epithelial-to-mesenchymal transition, a cellular state associated with increased tumor aggressiveness. Consistent with its activation in clinical CRPC, tumors driven by Notch1 intracellular domain in combination with multiple pathways altered in prostate cancer are metastatic and resistant to androgen deprivation. Our study provides functional evidence that the Notch1 signaling axis synergizes with alternative pathways in promoting metastatic CRPC and may represent a new therapeutic target for advanced prostate cancer.
Keywords: Notch1; cancer; prostate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







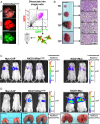
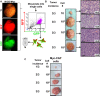
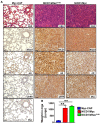
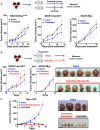
Comment in
-
Re: Activation of Notch1 Synergizes with Multiple Pathways in Promoting Castration-Resistant Prostate Cancer.J Urol. 2017 Mar;197(3 Pt 1):700-702. doi: 10.1016/j.juro.2016.12.030. Epub 2016 Dec 16. J Urol. 2017. PMID: 28208520 No abstract available.
-
Re: Activation of Notch1 Synergizes with Multiple Pathways in Promoting Castration-Resistant Prostate Cancer.J Urol. 2017 Jun;197(6):1458-1459. doi: 10.1016/j.juro.2017.03.013. Epub 2017 Mar 11. J Urol. 2017. PMID: 28505901 No abstract available.
References
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. - PubMed
-
- Kantoff PW, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. - PubMed
-
- Tannock IF, et al. TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. - PubMed
-
- Wang XD, et al. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol. 2006;290(1):66–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

