A Randomized, Controlled, Observer-Blinded Phase 1 Study of the Safety and Immunogenicity of a Respiratory Syncytial Virus Vaccine With or Without Alum Adjuvant
- PMID: 27694633
- PMCID: PMC5225248
- DOI: 10.1093/infdis/jiw453
A Randomized, Controlled, Observer-Blinded Phase 1 Study of the Safety and Immunogenicity of a Respiratory Syncytial Virus Vaccine With or Without Alum Adjuvant
Abstract
Background: Respiratory syncytial virus (RSV) is a leading cause of childhood bronchiolitis and pneumonia, particularly in early infancy. Immunization of pregnant women could boost preexisting immune responses, providing passive protection to newborns through placental transfer of anti-RSV antibody.
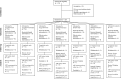
Methods: In this first-in-humans clinical trial of a purified recombinant RSV protein F vaccine engineered to preferentially maintain prefusion conformation (RSV-PreF), 128 healthy men 18-44 years old were randomized to one dose of a RSV-PreF vaccine containing 10, 30, or 60 µg of RSV-PreF antigen, with or without alum adjuvant, or control, and followed for one year for safety and immunogenicity outcomes.
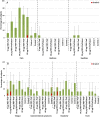
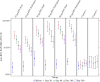
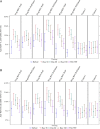
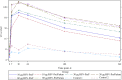
Results: Injection site pain was the most common adverse event, reported by up to 81.3% of participants. The highest RSV neutralizing antibody responses were in the 30 µg RSV-PreF/alum, 60 µg RSV-PreF/alum, and 60 µg RSV-PreF/nonadjuvant groups. Responses were evident on day 7, and 30 days after vaccination these participants had RSV-A neutralizing antibody titers of ≥1:512, and >70% had titers of 1:1024, with titers increasing by 3.2-4.9 fold. Responses remained high on day 60 but waned on days 180 and 360.
Conclusions: The RSV-PreF vaccine elicited rapid RSV neutralizing antibody responses in healthy young men, with an acceptable adverse event profile.
Keywords: maternal immunization; respiratory syncytial virus; vaccine; vaccine safety and immunogenicity.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Vaccines Against Respiratory Syncytial Virus: The Time Has Come.J Infect Dis. 2017 Jan 1;215(1):4-7. doi: 10.1093/infdis/jiw455. Epub 2016 Sep 29. J Infect Dis. 2017. PMID: 27694634 No abstract available.
References
-
- Langley JM, LeBlanc JC, Smith B, Wang EE. Increasing incidence of hospitalization for bronchiolitis among Canadian children, 1980-2000. J Infect Dis 2003; 188:1764–7. - PubMed
-
- Englund JA. Maternal immunization—promises and concerns. Vaccine 2015; 33:6372–3. - PubMed
-
- World Health Organization (WHO). The Global Vaccine and Immunization Research Forum (GVIRF). 2015. http://www.who.int/immunization/research/forums_and_initiatives/gvirf/en/ Accessed 12 October 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

