Zika viral dynamics and shedding in rhesus and cynomolgus macaques
- PMID: 27694931
- PMCID: PMC5293594
- DOI: 10.1038/nm.4206
Zika viral dynamics and shedding in rhesus and cynomolgus macaques
Erratum in
-
Corrigendum: Zika viral dynamics and shedding in rhesus and cynomolgus macaques.Nat Med. 2017 Feb 7;23(2):264. doi: 10.1038/nm0217-264b. Nat Med. 2017. PMID: 28170376 No abstract available.
Abstract
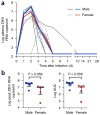
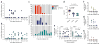
Infection with Zika virus has been associated with serious neurological complications and fetal abnormalities. However, the dynamics of viral infection, replication and shedding are poorly understood. Here we show that both rhesus and cynomolgus macaques are highly susceptible to infection by lineages of Zika virus that are closely related to, or are currently circulating in, the Americas. After subcutaneous viral inoculation, viral RNA was detected in blood plasma as early as 1 d after infection. Viral RNA was also detected in saliva, urine, cerebrospinal fluid (CSF) and semen, but transiently in vaginal secretions. Although viral RNA during primary infection was cleared from blood plasma and urine within 10 d, viral RNA was detectable in saliva and seminal fluids until the end of the study, 3 weeks after the resolution of viremia in the blood. The control of primary Zika virus infection in the blood was correlated with rapid innate and adaptive immune responses. We also identified Zika RNA in tissues, including the brain and male and female reproductive tissues, during early and late stages of infection. Re-infection of six animals 45 d after primary infection with a heterologous strain resulted in complete protection, which suggests that primary Zika virus infection elicits protective immunity. Early invasion of Zika virus into the nervous system of healthy animals and the extent and duration of shedding in saliva and semen underscore possible concern for additional neurologic complications and nonarthropod-mediated transmission in humans.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
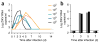


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

