Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice
- PMID: 27694932
- PMCID: PMC5503150
- DOI: 10.1038/nm.4192
Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice
Abstract
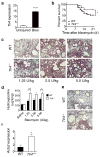
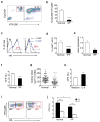
Successful recovery from lung injury requires the repair and regeneration of alveolar epithelial cells to restore the integrity of gas-exchanging regions within the lung and preserve organ function. Improper regeneration of the alveolar epithelium is often associated with severe pulmonary fibrosis, the latter of which involves the recruitment and activation of fibroblasts, as well as matrix accumulation. Type 2 alveolar epithelial cells (AEC2s) are stem cells in the adult lung that contribute to the lung repair process. The mechanisms that regulate AEC2 renewal are incompletely understood. We provide evidence that expression of the innate immune receptor Toll-like receptor 4 (TLR4) and the extracellular matrix glycosaminoglycan hyaluronan (HA) on AEC2s are important for AEC2 renewal, repair of lung injury and limiting the extent of fibrosis. Either deletion of TLR4 or HA synthase 2 in surfactant-protein-C-positive AEC2s leads to impaired renewal capacity, severe fibrosis and mortality. Furthermore, AEC2s from patients with severe pulmonary fibrosis have reduced cell surface HA and impaired renewal capacity, suggesting that HA and TLR4 are key contributors to lung stem cell renewal and that severe pulmonary fibrosis is the result of distal epithelial stem cell failure.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Innate immune signaling and stem cell renewal in idiopathic pulmonary fibrosis.Nat Med. 2016 Nov 8;22(11):1210-1212. doi: 10.1038/nm.4230. Nat Med. 2016. PMID: 27824820 No abstract available.
References
-
- Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. - PubMed
-
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

