Glycine triggers a non-ionotropic activity of GluN2A-containing NMDA receptors to confer neuroprotection
- PMID: 27694970
- PMCID: PMC5046082
- DOI: 10.1038/srep34459
Glycine triggers a non-ionotropic activity of GluN2A-containing NMDA receptors to confer neuroprotection
Abstract
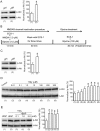
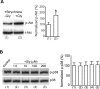
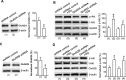
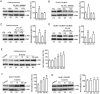
Ionotropic activation of NMDA receptors (NMDARs) requires agonist glutamate and co-agonist glycine. Here we show that glycine enhances the activation of cell survival-promoting kinase Akt in cultured cortical neurons in which both the channel activity of NMDARs and the glycine receptors are pre-inhibited. The effect of glycine is reduced by shRNA-mediated knockdown of GluN2A subunit-containing NMDARs (GluN2ARs), suggesting that a non-ionotropic activity of GluN2ARs mediates glycine-induced Akt activation. In support of this finding, glycine enhances Akt activation in HEK293 cells over-expressing GluN2ARs. The effect of glycine on Akt activation is sensitive to the antagonist of glycine-GluN1 binding site. As a functional consequence, glycine protects against excitotoxicity-induced neuronal death through the non-ionotropic activity of GluN2ARs and the neuroprotective effect is attenuated by Akt inhibition. Thus, this study reveals an unexpected role of glycine in eliciting a non-ionotropic activity of GluN2ARs to confer neuroprotection via Akt activation.
Figures

References
-
- Dingledine R., Borges K., Bowie D. & Traynelis S. F. The glutamate receptor ion channels. Pharmacol Rev 51, 7–61 (1999). - PubMed
-
- Monyer H. et al.. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256, 1217–1221 (1992). - PubMed
-
- Johnson J. W. & Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325, 529–531 (1987). - PubMed
-
- Malenka R. C. & Nicoll R. A. Long-term potentiation–a decade of progress? Science 285, 1870–1874 (1999). - PubMed
-
- Barria A. & Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

