Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression
- PMID: 27695003
- PMCID: PMC5215903
- DOI: 10.1038/ni.3577
Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression
Abstract
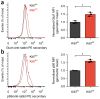
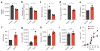
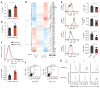
CD4+ effector T cells (Teff cells) and regulatory T cells (Treg cells) undergo metabolic reprogramming to support proliferation and immunological function. Although signaling via the lipid kinase PI(3)K (phosphatidylinositol-3-OH kinase), the serine-threonine kinase Akt and the metabolic checkpoint kinase complex mTORC1 induces both expression of the glucose transporter Glut1 and aerobic glycolysis for Teff cell proliferation and inflammatory function, the mechanisms that regulate Treg cell metabolism and function remain unclear. We found that Toll-like receptor (TLR) signals that promote Treg cell proliferation increased PI(3)K-Akt-mTORC1 signaling, glycolysis and expression of Glut1. However, TLR-induced mTORC1 signaling also impaired Treg cell suppressive capacity. Conversely, the transcription factor Foxp3 opposed PI(3)K-Akt-mTORC1 signaling to diminish glycolysis and anabolic metabolism while increasing oxidative and catabolic metabolism. Notably, Glut1 expression was sufficient to increase the number of Treg cells, but it reduced their suppressive capacity and Foxp3 expression. Thus, inflammatory signals and Foxp3 balance mTORC1 signaling and glucose metabolism to control the proliferation and suppressive function of Treg cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

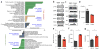
Comment in
-
Immunology: Metabolic changes modify Treg cell function.Nat Rev Rheumatol. 2016 Oct 21;12(11):621. doi: 10.1038/nrrheum.2016.172. Nat Rev Rheumatol. 2016. PMID: 27765952 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

