Diversity Takes Shape: Understanding the Mechanistic and Adaptive Basis of Bacterial Morphology
- PMID: 27695035
- PMCID: PMC5047622
- DOI: 10.1371/journal.pbio.1002565
Diversity Takes Shape: Understanding the Mechanistic and Adaptive Basis of Bacterial Morphology
Abstract
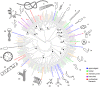
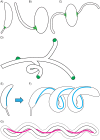
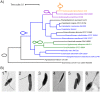
The modern age of metagenomics has delivered unprecedented volumes of data describing the genetic and metabolic diversity of bacterial communities, but it has failed to provide information about coincident cellular morphologies. Much like metabolic and biosynthetic capabilities, morphology comprises a critical component of bacterial fitness, molded by natural selection into the many elaborate shapes observed across the bacterial domain. In this essay, we discuss the diversity of bacterial morphology and its implications for understanding both the mechanistic and the adaptive basis of morphogenesis. We consider how best to leverage genomic data and recent experimental developments in order to advance our understanding of bacterial shape and its functional importance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

