Production, Quality Control, Stability and Pharmacotoxicity of a Malaria Vaccine Comprising Three Highly Similar PfAMA1 Protein Molecules to Overcome Antigenic Variation
- PMID: 27695087
- PMCID: PMC5047445
- DOI: 10.1371/journal.pone.0164053
Production, Quality Control, Stability and Pharmacotoxicity of a Malaria Vaccine Comprising Three Highly Similar PfAMA1 Protein Molecules to Overcome Antigenic Variation
Abstract
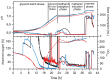
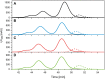
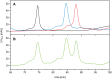
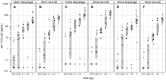
Plasmodium falciparum apical membrane antigen 1 (PfAMA1) is a leading asexual blood stage vaccine candidate for malaria. In preparation for clinical trials, three Diversity Covering (DiCo) PfAMA1 ectodomain proteins, designed to overcome the intrinsic polymorphism that is present in PfAMA1, were produced under Good Manufacturing Practice (GMP) in Pichia pastoris. Using identical methodology, the 3 strains were cultivated in 70-L scale fed-batch fermentations and PfAMA1-DiCos were purified by two chromatography steps, an ultrafiltration/diafiltration procedure and size exclusion chromatography, resulting in highly pure (>95%) PfAMA1-DiCo1, PfAMA1 DiCo2 and PfAMA1 DiCo3, with final yields of 1.8, 1.9 and 1.3 gram, respectively. N-terminal determinations showed that approximately 50% of each of the proteins lost 12 residues from their N-terminus, in accordance with SDS-PAGE (2 main bands) and MS-data. Under reducing conditions a site of limited proteolytic cleavage within a disulphide bonded region became evident. The three proteins quantitatively bound to the mAb 4G2 that recognizes a conformational epitope, suggesting proper folding of the proteins. The lyophilized Drug Product (1:1:1 mixture of PfAMA1-DiCo1, DiCo2, DiCo3) fulfilled all pre-set release criteria (appearance, dissolution rate, identity, purity, protein content, moisture content, sub-visible particles, immuno-potency (after reconstitution with adjuvant), abnormal toxicity, sterility and endotoxin), was stable in accelerated and real-time stability studies at -20°C for over 24 months. When formulated with adjuvants selected for clinical phase I evaluation, the Drug Product did not show adverse effect in a repeated-dose toxicity study in rabbits. The Drug Product has entered a phase Ia/Ib clinical trial.
Conflict of interest statement
I have read the journal's policy and four of the authors of this manuscript (BWF, AWT, CHMK and EJR) have the following competing interest: They hold a patent on the products described in this paper (patent US8741305 B2 (Jun 3, 2014), entitled "Protein Composition for Inducing an Immune Response in a Vertebrate Comprising a Plurality of Protein Variants Covering the Heterogeneity of a Single Antigen, AMA1". This does not alter our adherence to PLOS ONE policies on sharing data and materials. One of the authors (RB) is employed by a commercial company (Nova Laboratories Ltd.). This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Production, quality control, stability and pharmacotoxicity of cGMP-produced Plasmodium falciparum AMA1 FVO strain ectodomain expressed in Pichia pastoris.Vaccine. 2008 Nov 11;26(48):6143-50. doi: 10.1016/j.vaccine.2008.08.055. Epub 2008 Sep 17. Vaccine. 2008. PMID: 18804135
-
Phase 1 randomized controlled trial to evaluate the safety and immunogenicity of recombinant Pichia pastoris-expressed Plasmodium falciparum apical membrane antigen 1 (PfAMA1-FVO [25-545]) in healthy Malian adults in Bandiagara.Malar J. 2016 Aug 30;15(1):442. doi: 10.1186/s12936-016-1466-4. Malar J. 2016. PMID: 27577237 Free PMC article. Clinical Trial.
-
A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits.Infect Immun. 2008 Jun;76(6):2660-70. doi: 10.1128/IAI.00170-08. Epub 2008 Mar 31. Infect Immun. 2008. PMID: 18378635 Free PMC article.
-
Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum.Hum Vaccin. 2010 Jan;6(1):39-53. doi: 10.4161/hv.6.1.10712. Epub 2010 Jan 19. Hum Vaccin. 2010. PMID: 20061790 Review.
-
Pfs230: from malaria transmission-blocking vaccine candidate toward function.Parasite Immunol. 2003 Jul;25(7):351-9. doi: 10.1046/j.1365-3024.2003.00643.x. Parasite Immunol. 2003. PMID: 14521577 Review. No abstract available.
Cited by
-
Plasmodium falciparum AMA1 and CSP antigen diversity in parasite isolates from southern Ghana.Front Cell Infect Microbiol. 2024 May 13;14:1375249. doi: 10.3389/fcimb.2024.1375249. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38808064 Free PMC article.
-
Variations in the quality of malaria-specific antibodies with transmission intensity in a seasonal malaria transmission area of Northern Ghana.PLoS One. 2017 Sep 25;12(9):e0185303. doi: 10.1371/journal.pone.0185303. eCollection 2017. PLoS One. 2017. PMID: 28945794 Free PMC article.
-
Production, quality control, stability, and potency of cGMP-produced Plasmodium falciparum RH5.1 protein vaccine expressed in Drosophila S2 cells.NPJ Vaccines. 2018 Aug 17;3:32. doi: 10.1038/s41541-018-0071-7. eCollection 2018. NPJ Vaccines. 2018. PMID: 30131879 Free PMC article.
-
Plasmodium vivax ligand-receptor interaction: PvAMA-1 domain I contains the minimal regions for specific interaction with CD71+ reticulocytes.Sci Rep. 2017 Aug 30;7(1):9616. doi: 10.1038/s41598-017-10025-6. Sci Rep. 2017. PMID: 28855657 Free PMC article.
References
-
- WHO. World malaria report: 2014. Geneva: World Health Organization, 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

