Borrelia burgdorferi Induces TLR2-Mediated Migration of Activated Dendritic Cells in an Ex Vivo Human Skin Model
- PMID: 27695100
- PMCID: PMC5047638
- DOI: 10.1371/journal.pone.0164040
Borrelia burgdorferi Induces TLR2-Mediated Migration of Activated Dendritic Cells in an Ex Vivo Human Skin Model
Abstract
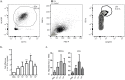
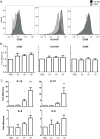
Borrelia burgdorferi is transmitted into the skin of the host where it encounters and interacts with two dendritic cell (DC) subsets; Langerhans cells (LCs) and dermal DCs (DDCs). These cells recognize pathogens via pattern recognition receptors, mature and migrate out of the skin into draining lymph nodes, where they orchestrate adaptive immune responses. In order to investigate the response of skin DCs during the early immunopathogenesis of Lyme borreliosis, we injected B. burgdorferi intradermally into full-thickness human skin and studied the migration of DCs out of the skin, the activation profile and phenotype of migrated cells. We found a significant increase in the migration of LCs and DDCs in response to B. burgdorferi. Notably, migration was prevented by blocking TLR2. DCs migrated from skin inoculated with higher numbers of spirochetes expressed significantly higher levels of CD83 and produced pro-inflammatory cytokines. No difference was observed in the expression of HLA-DR, CD86, CD38, or CCR7. To conclude, we have established an ex vivo human skin model to study DC-B. burgdorferi interactions. Using this model, we have demonstrated that B. burgdorferi-induced DC migration is mediated by TLR2. Our findings underscore the utility of this model as a valuable tool to study immunity to spirochetal infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, et al. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. Journal of immunology. 1999;163(5):2382–6. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

