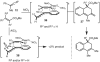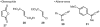An unexpected Lewis acid catalyzed Diels-Alder cycloaddition of aryl allenes and acrylates
- PMID: 27695139
- PMCID: PMC5038599
- DOI: 10.1016/j.tet.2016.02.028
An unexpected Lewis acid catalyzed Diels-Alder cycloaddition of aryl allenes and acrylates
Abstract
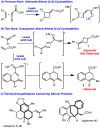
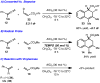
An unexpected [4+2] cycloaddition of aryl allenes and simple acrylate derivatives is reported. This process functions well with a variety of allenes and acrylates to generate bi- and tricyclic dihydronaphthalene derivatives through a nonconventional bond disconnection.
Keywords: Acrylates; Allenes; Cycloaddition; Diels-Alder; Lewis-acid.
Figures
References
-
-
For recent reviews see: Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem, Int Ed. 2002;41:1668.Heravi MM, Vavsari VF. RSC Adv. 2015;5:50890.Jiang X, Wang R. Chem Rev. 2013;113:5515.
-
-
- Conner ML, Xu Y, Brown MK. J Am Chem Soc. 2015;137:3482. - PubMed
- Rasik CM, Brown MK. Angew Chem, Int Ed. 2014;53:14522. - PubMed
- Rasik CM, Hong YJ, Tantillo DJ, Brown MK. Org Lett. 2014;16:5168. - PubMed
- Rasik C, Brown M. Synlett. 2014:760.
- Rasik CM, Brown MK. J Am Chem Soc. 2013;135:1673. - PubMed
-
- Snider BB, Ron E. J Org Chem. 1986;51:3643.
- Hoffmann H, Ismail ZM, Weber A. Tetrahedron Lett. 1981;22:1953.
- Snider BB, Spindell DK. J Org Chem. 1980;45:5017.
-
- Modern Allene Chemistry. Wiley-VCH; Weinheim, Germany: 2004.
- Okamura WH, Curtin ML. Synlett. 1990:1.
-
- Liu L, Li W, Sasaki T, Asada Y, Koike KJ. Nat Med. 2010;64:496. - PubMed
- Lazerwith SE, Johnson TW, Corey E. J Org Lett. 2000;2:2389. - PubMed
- Looks SA, Fenical W, Jacobs RS, Clardy J. Proc Natl Acad Sci. 1986;83:6238. - PMC - PubMed
- Looks SA, Fenical W, Matsumoto GK, Clardy J. J Org Chem. 1986;51:5140.
- Tanaka JI, Ogawa N, Liang J, Higa T, Gravalos DG. Tetrahedron. 1993;49:811.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources