Elucidation of Flavonoids from Carissa congesta, Polyalthia longifolia, and Benincasa hispida Plant Extracts by Hyphenated Technique of Liquid Chromatography-mass Spectroscopy
- PMID: 27695269
- PMCID: PMC5004520
- DOI: 10.4103/0974-8490.186578
Elucidation of Flavonoids from Carissa congesta, Polyalthia longifolia, and Benincasa hispida Plant Extracts by Hyphenated Technique of Liquid Chromatography-mass Spectroscopy
Abstract
Background: Carissa congesta (CC), Polyalthia longifolia (PL), and Benincasa hispida (BH) are economically important plants.
Objective: Current research encompasses identification of quercetin and rutin and their analogues by liquid chromatography-mass spectroscopy (LC-MS) from the selected plant species.
Materials and methods: Fresh roots, leaves, and seeds of CC, PL, and BH plants respectively were shade-dried followed by extraction and elucidation of rutin and quercetin by LC-MS.
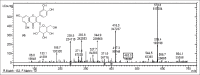
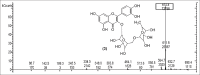
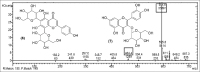
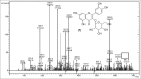
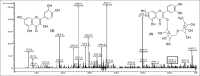
Results: Structural elucidation of CC, PL, and BH extracts revealed the presence of flavonoids such as quercetin (m/z 301) and rutin (m/z 610) as the parent ions along with presence of close analogues such as quercetin-O-hexoside, Vicenin 2, quercetin-3-O-xyloside/arabinoside, and quercetin-3-O-glucoside were identified as fragments.
Conclusions: Thus, CC, PL, and BH extracts revealed the presence of flavonoids belonging to the class of flavonols such as rutin and quercetin.
Summary: Quercetin and rutin were identified from CC roots, PL leaves and BH seeds by liquid chromatography-mass spectroscopy.Quercetin was characterized at (m/z 301) and rutin (m/z 610) as the parent ion peaks.Analogues such as quercetin-O-hexoside, Vicenin 2 and quercetin-3-O-glucoside were identified as fragments.
Keywords: Benincasa hispida; Carissa congesta; Liquid chromatography; Mass spectroscopy; Polyalthia longifolia; Quercetin; Rutin.
Figures






References
-
- Shropshire W., JR . Action spectroscopy. In: Mitrakos K, Shropshire W Jr, editors. Phytochrome. London: Academic Press; 1972. pp. 161–81.
-
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–53. - PubMed
-
- Iwashina T. The structure and distribution of the flavonoids in plants. J Plant Res. 2000;113:287–99.
-
- Shahidi F. Chemistry, Health Effects and Practical Applications. Champaign, Illinois: AOCS Press; 1997. Flavonoids as antioxidants. Natural Antioxidants; p. 174.
LinkOut - more resources
Full Text Sources
Other Literature Sources
