Effectiveness and safety of vedolizumab for treatment of Crohn's disease: a systematic review and meta-analysis
- PMID: 27695501
- PMCID: PMC5016590
- DOI: 10.5114/aoms.2016.61915
Effectiveness and safety of vedolizumab for treatment of Crohn's disease: a systematic review and meta-analysis
Abstract
Introduction: The aim of this systematic review (SR) and meta-analysis was to assess the efficacy and safety of vedolizumab in the treatment of Crohn's disease (CD).
Material and methods: A systematic literature search was conducted in Medline/PubMed, Embase and Cochrane Library until 25 January, 2015. Included studies were critically appraised according to the PRISMA protocol. Assessment in specified subgroups of CD patients and meta-analysis with Revman software were performed.
Results: Two randomized controlled trial (RCTs) were included in a meta-analysis for the induction phase of therapy: GEMINI II and GEMINI III. The clinical response was significantly higher for patients who received vedolizumab compared to placebo in the general population (risk benefit (RB) = 1.48; p = 0.0006) and in both analyzed subgroups: patients with previous failure of anti-TNFs treatment (RB = 1.51; p = 0.006) and patients naive to earlier anti-TNFs (RB = 1.41; p = 0.001). The clinical remission in the general population and subpopulation of TNF-antagonist naive patients was significantly higher for patients who received vedolizumab compared to placebo (RB = 1.77; p = 0.003; RB = 2.29; p = 0.0004; respectively). Meta-analysis for adverse events, serious adverse events (SAEs) and serious infections, revealed that vedolizumab was as safe as placebo in the induction phase of therapy.
Conclusions: The clinical response was significantly higher for patients who received vedolizumab in the general population and in both analyzed subgroups of patients. The clinical remission in the general population and subpopulation of TNF-antagonist naive patients was significantly higher for vedolizumab, but no significant differences were revealed in the subgroup of patients with previous TNF antagonist failure.
Keywords: Crohn's disease; MLN-002; meta-analysis; systematic review; vedolizumab.
Figures
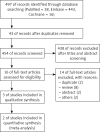
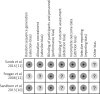



References
-
- Assadsangabi A, Lobo AJ. Diagnosing and managing inflammatory bowel disease. Practitioner. 2013;257:13–8. - PubMed
-
- Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–605. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. - PubMed
-
- Yonekawa K, Harlan JM. Promises and limitations of targeting adhesion molecules for therapy. In: Ley K, editor. Adhesion molecules: function and inhibition. Basel (Switzerland): Birkh user Verlag AG; 2007. pp. 289–330.
-
- Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the madcam-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–28. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
