Cell Intrinsic Factors Modulate the Effects of IFNγ on the Development of Chlamydia trachomatis
- PMID: 27695641
- PMCID: PMC5040356
- DOI: 10.4172/2155-9597.1000282
Cell Intrinsic Factors Modulate the Effects of IFNγ on the Development of Chlamydia trachomatis
Abstract
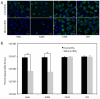
Chlamydia trachomatis is an obligate intracellular bacterial pathogen that cannot synthesize several amino acids, including tryptophan. Rather, C. trachomatis acquires these essential metabolites from its human host cell. Chlamydial dependence on host-provided tryptophan underlies a major host defense mechanism against the bacterium; namely, the induction of the host tryptophan-catabolizing enzyme, indoleamine 2,3- dioxygenase (IDO1) by interferon gamma (IFNγ), which leads to eradication of C. trachomatis by tryptophan starvation. For this reason, IFNγ is proposed to be the major host protective cytokine against genital C. trachomatis infections. The protective effect of IFNγ against C. trachomatis can be recapitulated in vitro using epithelial cell-lines such as the cervical carcinoma derived cell-line Hela, the Hela subclone HEp-2, and the cervical carcinoma derived cell-line ME180. Addition of IFNγ to these cells infected with C. trachomatis results in a strong bactericidal or bacteriostatic effect dependent on the concentration of IFNγ administered. Unlike Hela, HEp-2, and ME180, there are other human epithelial, or epithelial-like cell-lines where administration of IFNγ does not affect chlamydial replication, although they express the IFNγ receptor (IFNGR). In this report, we have characterized the mechanisms that underlie this dichotomy using the cell-lines C33A and 293. Akin to Hela, C33A is derived from a human cervical carcinoma, while 293 cells were produced by transfection of adenovirus type 5 DNA into embryonic kidney cells. We demonstrate that although IFNGR is expressed at high levels in C33A cells, its ligation by IFNγ does not result in STAT1 phosphorylation, an essential step for activation of the IDO1 promoter. Our results indicate that although the IFNγ-dependent signaling cascade is intact in 293 cells; the IDO1 promoter is not activated in these cells because it is epigenetically silenced, most likely by DNA methylation. Because polymorphisms in IFNγ, IFNGR, and the IDO1 promoter are known to affect other human infections or diseased states, our results indicate that the effect of allelic differences in these genes and the pathways they activate should be evaluated for their effect on C. trachomatis pathology.
Figures




References
-
- Schachter J. Chlamydial infections (first of three parts) N Engl J Med. 1978;298:428–435. - PubMed
-
- Fehlner-Gardiner C, Roshick C, Carlson JH, Hughes S, Belland RJ, et al. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J Biol Chem. 2002;277:26893–26903. - PubMed
-
- Thylefors B. Global trachoma control past, present and future approaches. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique. 1995;72:13–80. - PubMed
-
- Schachter J, Moncada J, Dawson CR, Sheppard J, Courtright P, et al. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988;158:1347–1352. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
