A neurodegenerative perspective on mitochondrial optic neuropathies
- PMID: 27696015
- PMCID: PMC5106504
- DOI: 10.1007/s00401-016-1625-2
A neurodegenerative perspective on mitochondrial optic neuropathies
Abstract
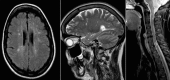
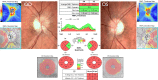
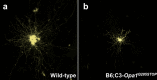
Mitochondrial optic neuropathies constitute an important cause of chronic visual morbidity and registrable blindness in both the paediatric and adult population. It is a genetically heterogeneous group of disorders caused by both mitochondrial DNA (mtDNA) mutations and a growing list of nuclear genetic defects that invariably affect a critical component of the mitochondrial machinery. The two classical paradigms are Leber hereditary optic neuropathy (LHON), which is a primary mtDNA disorder, and autosomal dominant optic atrophy (DOA) secondary to pathogenic mutations within the nuclear gene OPA1 that encodes for a mitochondrial inner membrane protein. The defining neuropathological feature is the preferential loss of retinal ganglion cells (RGCs) within the inner retina but, rather strikingly, the smaller calibre RGCs that constitute the papillomacular bundle are particularly vulnerable, whereas melanopsin-containing RGCs are relatively spared. Although the majority of patients with LHON and DOA will present with isolated optic nerve involvement, some individuals will also develop additional neurological complications pointing towards a greater vulnerability of the central nervous system (CNS) in susceptible mutation carriers. These so-called "plus" phenotypes are mechanistically important as they put the loss of RGCs within the broader perspective of neuronal loss and mitochondrial dysfunction, highlighting common pathways that could be modulated to halt progressive neurodegeneration in other related CNS disorders. The management of patients with mitochondrial optic neuropathies still remains largely supportive, but the development of effective disease-modifying treatments is now within tantalising reach helped by major advances in drug discovery and delivery, and targeted genetic manipulation.
Keywords: Dominant optic atrophy; Leber hereditary optic neuropathy; Mitochondrial diseases; Neurodegenerative diseases; OPA1; Retinal ganglion cell.
Conflict of interest statement
Compliance with ethical standards Financial disclosures PYWM holds a consultancy agreement with GenSight Biologics. VC holds consultancy agreements with GenSight Biologics, Santhera Pharmaceuticals, Stealth BioTherapeutics and Edison Pharmaceuticals.
Figures


References
-
- Adams JH, Blackwood W, Wilson J. Further clinical and pathological observations on Leber’s optic atrophy. Brain. 1966;89:15–26. - PubMed
-
- Alavi MV, Bette S, Schimpf S, Schuettauf F, Schraermeyer U, Wehrl HF, Ruttiger L, Beck SC, Tonagel F, Pichler BJ, et al. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130:1029–1042. - PubMed
-
- Alavi MV, Fuhrmann N, Nguyen HP, Yu-Wai-Man P, Heiduschka P, Chinnery PF, Wissinger B. Subtle neurological and metabolic abnormalities in an Opa1 mouse model of autosomal dominant optic atrophy. Exp Neurol. 2009;220:404–409. - PubMed
-
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

