All-trans retinoic acid as adjunct to intensive treatment in younger adult patients with acute myeloid leukemia: results of the randomized AMLSG 07-04 study
- PMID: 27696203
- PMCID: PMC5093206
- DOI: 10.1007/s00277-016-2810-z
All-trans retinoic acid as adjunct to intensive treatment in younger adult patients with acute myeloid leukemia: results of the randomized AMLSG 07-04 study
Abstract
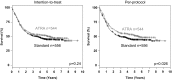
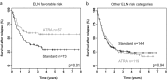
The aim of this clinical trial was to evaluate the impact of all-trans retinoic acid (ATRA) in combination with chemotherapy and to assess the NPM1 status as biomarker for ATRA therapy in younger adult patients (18-60 years) with acute myeloid leukemia (AML). Patients were randomized for intensive chemotherapy with or without open-label ATRA (45 mg/m2, days 6-8; 15 mg/m2, days 9-21). Two cycles of induction therapy were followed by risk-adapted consolidation with high-dose cytarabine or allogeneic hematopoietic cell transplantation. Due to the open label character of the study, analysis was performed on an intention-to-treat (ITT) and a per-protocol (PP) basis. One thousand one hundred patients were randomized (556, STANDARD; 544, ATRA) with 38 patients treated vice versa. Median follow-up for survival was 5.2 years. ITT analyses revealed no difference between ATRA and STANDARD for the total cohort and for the subset of NPM1-mutated AML with respect to event-free (EFS; p = 0.93, p = 0.17) and overall survival (OS; p = 0.24 and p = 0.32, respectively). Pre-specified PP analyses revealed better EFS in NPM1-mutated AML (p = 0.05) and better OS in the total cohort (p = 0.03). Explorative subgroup analyses on an ITT basis revealed better OS (p = 0.05) in ATRA for genetic low-risk patients according to ELN recommendations. The clinical trial is registered at clinicaltrialsregister.eu (EudraCT Number: 2004-004321-95).
Keywords: Acute myeloid leukemia; All-trans retinoic acid; Nucleophosmin-1.
Conflict of interest statement
Compliance with ethical standards Conflicts of interest Authors indicated no potential conflict of interest. Authors’ contribution Conception and Design: Richard F. Schlenk, Michael Lübbert, Hartmut Döhner Provision of study materials or patients: Richard F. Schlenk, Michael Lübbert, Jürgen Krauter, Thomas Kindler, Hans Martin, Helmut R. Salih, Andrea Kündgen, Heinz-A. Horst, Peter Brossart, Katharina Götze, David Nachbaur, Mohammed Wattad, Claus-Henning Köhne, Walter Fiedler, Martin Bentz, Gerald Wulf, Gerhard Held, Bernd Hertenstein, Hans Salwender, Verena I Gaidzik, Brigitte Schlegelberger, Konstanze Döhner, Arnold Ganser, Hartmut Döhner. Collection and assembly of data: Richard F. Schlenk, Alexander Lamparter, Daniela Weber. Data analysis and interpretation: Richard F. Schlenk, Alexander Lamparter, Axel Benner, Hartmut Döhner. Manuscript writing: Richard F. Schlenk, Hartmut Döhner. Final approval of manuscript: Richard F. Schlenk, Michael Lübbert, Axel Benner, Alexander Lamparter, Jürgen Krauter, Thomas Kindler, Hans Martin, Helmut R. Salih, Andrea Kündgen, Heinz-A. Horst, Peter Brossart, Katharina Götze, David Nachbaur, Mohammed Wattad, Claus-Henning Köhne, Walter Fiedler, Martin Bentz, Gerald Wulf, Gerhard Held, Bernd Hertenstein, Hans Salwender, Verena I Gaidzik, Brigitte Schlegelberger, Daniela Weber, Konstanze Döhner, Arnold Ganser, Hartmut Döhner.
Figures

References
-
- Hu ZB, Minden MD, McCulloch EA. Direct evidence for the participation of bcl-2 in the regulation by retinoic acid of the Ara-C sensitivity of leukemic stem cells. Leukemia. 1995;9:1667–73. - PubMed
-
- Yang GS, Minden MD, McCulloch EA. Influence of schedule on regulated sensitivity of AML blasts to cytosine arabinoside. Leukemia. 1993;7:1012–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

