Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies
- PMID: 27696577
- PMCID: PMC5412924
- DOI: 10.1111/jdv.13990
Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies
Abstract
Background: Biologics are effective for the treatment of psoriasis. However, treatment outcomes may differ among biologic-naive patients and those switched from previous biological therapies.
Objectives: The study's objective was to investigate efficacy and safety of ixekizumab, a high-affinity anti-interleukin-17A antibody, in patients with psoriasis with and without previous exposure to biologics.
Methods: Data were integrated from the 12-week induction phase of two etanercept-controlled Phase III trials. Patients received 80 mg ixekizumab every 2 weeks (IXE Q2W; N = 736) or every 4 weeks (IXE Q4W; N = 733) following a 160-mg starting dose, or placebo (N = 361). Etanercept (50 mg twice weekly; N = 740) was administered as active control. Psoriasis Area and Severity Index (PASI) 75, PASI 90 and PASI 100 response rates at week 12 were evaluated in patients with or without previous exposure to biologics. Treatment effects were analysed with the Cochran-Mantel-Haenszel test stratified by study; missing values were imputed as non-response.
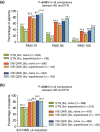
Results: Overall, 497 (19.3%) patients had prior exposure to biologics and 2073 (80.7%) were naive to biologic therapy. PASI 75 was achieved by 91.5% of biologic-experienced patients and 87.7% of biologic-naive patients for IXE Q2W, 76.2% and 82.2% for IXE Q4W, respectively, and 34.6% and 50.7%, respectively, for etanercept. Higher response rates favouring each ixekizumab dose over etanercept within subgroups were also seen regarding PASI 90 and PASI 100.
Conclusions: Contrary to etanercept, the efficacy of ixekizumab was similarly high in patients with and without previous exposure to biologics when administered 80 mg every 2 weeks.
© 2016 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures
References
-
- Menter A. The status of biologic therapies in the treatment of moderate to severe psoriasis. Cutis 2009; 84(4 Suppl): 14–24. - PubMed
-
- Menter A, Korman NJ, Elmets CA et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case‐based presentations and evidence‐based conclusions. J Am Acad Dermatol 2011; 65: 137–174. - PubMed
-
- Nast A, Boehncke WH, Mrowietz U et al German S3‐guidelines on the treatment of psoriasis vulgaris (short version). Arch Dermatol Res 2012; 304: 87–113. - PubMed
-
- Pathirana D, Ormerod AD, Saiag P et al European S3‐guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009; 23(Suppl 2): 1–70. - PubMed
-
- Smith CH, Anstey AV, Barker JN et al British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009; 161: 987–1019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

