In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis
- PMID: 27696751
- PMCID: PMC5329054
- DOI: 10.1002/art.39938
In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis
Abstract
Objective: Antiphospholipid syndrome (APS) is a leading acquired cause of thrombotic events. Although antiphospholipid antibodies have been shown to promote thrombosis in mice, the role of neutrophils has not been explicitly studied. The aim of this study was to characterize neutrophils in the context of a new model of antiphospholipid antibody-mediated venous thrombosis.
Methods: Mice were administered fractions of IgG obtained from patients with APS. At the same time, blood flow through the inferior vena cava was reduced by induction of stenosis. Resulting thrombi were characterized for size and neutrophil content. Circulating factors and the vessel wall were also assessed.
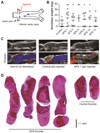
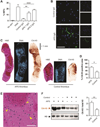
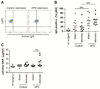
Results: As measured by both thrombus weight and thrombosis frequency, mice treated with IgG from patients with APS (APS IgG) demonstrated exaggerated thrombosis as compared with control IgG-treated mice. Thrombi in mice treated with APS IgG were enriched for citrullinated histone H3 (a marker of neutrophil extracellular traps [NETs]). APS IgG-treated mice also demonstrated elevated levels of circulating cell-free DNA and human IgG bound to the neutrophil surface. In contrast, circulating neutrophil numbers and markers of vessel wall activation were not appreciably different between APS IgG-treated mice and control mice. Treatment with either DNase (which dissolves NETs) or a neutrophil-depleting antibody reduced thrombosis in APS IgG-treated mice to the level in control mice.
Conclusion: These data support a mechanism whereby circulating neutrophils are primed by antiphospholipid antibodies to accelerate thrombosis. This line of investigation suggests new, immunomodulatory approaches for the treatment of APS.
© 2016, American College of Rheumatology.
Conflict of interest statement
The authors have no competing interests or conflicts to disclose.
Figures



References
-
- Gomez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. Journal of autoimmunity. 2014;48–49:20–25. - PubMed
-
- Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmunity reviews. 2014;13(9):917–930. - PubMed
-
- Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of "non-criteria" clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmunity reviews. 2015;14(5):401–414. - PubMed
-
- Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Seminars in thrombosis and hemostasis. 2012;38(4):305–321. - PubMed
-
- Pericleous C, Ruiz-Limon P, Romay-Penabad Z, Marin AC, Garza-Garcia A, Murfitt L, et al. Proof-of-concept study demonstrating the pathogenicity of affinity-purified IgG antibodies directed to domain I of beta2-glycoprotein I in a mouse model of anti-phospholipid antibody-induced thrombosis. Rheumatology. 2015;54(4):722–727. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

