Heparan Sulfate Regulates the Structure and Function of Osteoprotegerin in Osteoclastogenesis
- PMID: 27697839
- PMCID: PMC5104940
- DOI: 10.1074/jbc.M116.751974
Heparan Sulfate Regulates the Structure and Function of Osteoprotegerin in Osteoclastogenesis
Abstract
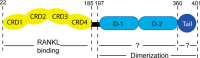
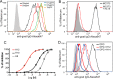
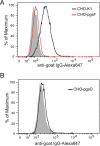
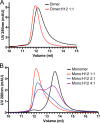
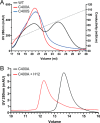
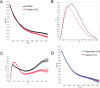
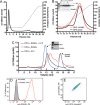
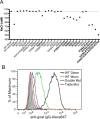
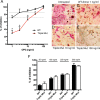
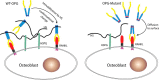
Osteoprotegerin (OPG), a decoy receptor secreted by osteoblasts, is a major negative regulator of bone resorption. It functions by neutralizing the receptor activator of nuclear factor κB ligand (RANKL), which plays a central role in promoting osteoclastogenesis. OPG is known to be a high-affinity heparan sulfate (HS)-binding protein. Presumably, HS could regulate the function of OPG and affect how it inhibits RANKL. However, the molecular detail of HS-OPG interaction remains poorly understood, which hinders our understanding of how HS functions in osteoclastogenesis. Here we report mapping of the HS-binding site of OPG. The HS-binding site, identified by mutagenesis study, consists of eight basic residues that are located mostly at the junction of the second death domain and the C-terminal domain. We further show that heparin-derived dodecasaccharide is able to induce dimerization of OPG monomers with a stoichiometry of 1:1. Small-angle X-ray scattering analysis revealed that upon binding of HS, OPG undergoes a dramatic conformational change, resulting in a more compact and less flexible structure. Importantly, we present here three lines of evidence that HS, OPG, and RANKL form a stable ternary complex. Using a HS binding-deficient OPG mutant, we further show that in an osteoblast/bone marrow macrophage co-culture system, immobilization of OPG by HS at the osteoblast cell surface substantially lowers the inhibitory threshold of OPG toward RANKL. These discoveries strongly suggest that HS plays an active role in regulating OPG-RANKL interaction and osteoclastogenesis.
Keywords: RANKL; conformational change; dimerization; heparan sulfate; osteoblast; osteoclastogenesis; osteoprotegerin; small-angle X-ray scattering (SAXS); ternary complex.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Antiresorptive activity of osteoprotegerin requires an intact heparan sulfate-binding site.Proc Natl Acad Sci U S A. 2020 Jul 21;117(29):17187-17194. doi: 10.1073/pnas.2005859117. Epub 2020 Jul 7. Proc Natl Acad Sci U S A. 2020. PMID: 32636266 Free PMC article.
-
Heparan sulfate selectively inhibits the collagenase activity of cathepsin K.Matrix Biol. 2024 May;129:15-28. doi: 10.1016/j.matbio.2024.03.005. Epub 2024 Mar 26. Matrix Biol. 2024. PMID: 38548090 Free PMC article.
-
Dimerization interface of osteoprotegerin revealed by hydrogen-deuterium exchange mass spectrometry.J Biol Chem. 2018 Nov 9;293(45):17523-17535. doi: 10.1074/jbc.RA118.004489. Epub 2018 Sep 25. J Biol Chem. 2018. PMID: 30254073 Free PMC article.
-
Action of RANKL and OPG for osteoclastogenesis.Crit Rev Eukaryot Gene Expr. 2009;19(1):61-72. doi: 10.1615/critreveukargeneexpr.v19.i1.30. Crit Rev Eukaryot Gene Expr. 2009. PMID: 19191757 Review.
-
Does the OPG/RANKL system contribute to the bone-vascular axis in chronic kidney disease? A systematic review.Adv Med Sci. 2017 Mar;62(1):52-64. doi: 10.1016/j.advms.2016.08.001. Epub 2017 Feb 9. Adv Med Sci. 2017. PMID: 28189120
Cited by
-
Heparan sulfate promotes TRAIL-induced tumor cell apoptosis.Elife. 2024 Jan 24;12:RP90192. doi: 10.7554/eLife.90192. Elife. 2024. PMID: 38265424 Free PMC article.
-
Mesenchymal Cell-Derived Juxtacrine Wnt1 Signaling Regulates Osteoblast Activity and Osteoclast Differentiation.J Bone Miner Res. 2019 Jun;34(6):1129-1142. doi: 10.1002/jbmr.3680. Epub 2019 Mar 7. J Bone Miner Res. 2019. PMID: 30690791 Free PMC article.
-
The Role of Heparan Sulfate in Bone Repair and Regeneration.Calcif Tissue Int. 2025 Jul 24;116(1):102. doi: 10.1007/s00223-025-01413-6. Calcif Tissue Int. 2025. PMID: 40705086 Free PMC article. Review.
-
Biochemical characterization of a disease-causing human osteoprotegerin variant.Sci Rep. 2022 Sep 10;12(1):15279. doi: 10.1038/s41598-022-19522-9. Sci Rep. 2022. PMID: 36088403 Free PMC article.
-
Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full-length OPG-fc in mitigating muscular dystrophy.Acta Neuropathol Commun. 2018 Apr 24;6(1):31. doi: 10.1186/s40478-018-0533-1. Acta Neuropathol Commun. 2018. PMID: 29699580 Free PMC article.
References
-
- Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 - PubMed
-
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., and Suda T. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 - PMC - PubMed
-
- Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H. L., et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 - PubMed
-
- Udagawa N., Takahashi N., Yasuda H., Mizuno A., Itoh K., Ueno Y., Shinki T., Gillespie M. T., Martin T. J., Higashio K., and Suda T. (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141, 3478–3484 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

