Nanoscopic compartmentalization of membrane protein motion at the axon initial segment
- PMID: 27697928
- PMCID: PMC5057285
- DOI: 10.1083/jcb.201603108
Nanoscopic compartmentalization of membrane protein motion at the axon initial segment
Abstract
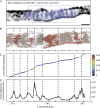
The axon initial segment (AIS) is enriched in specific adaptor, cytoskeletal, and transmembrane molecules. During AIS establishment, a membrane diffusion barrier is formed between the axonal and somatodendritic domains. Recently, an axonal periodic pattern of actin, spectrin, and ankyrin forming 190-nm-spaced, ring-like structures has been discovered. However, whether this structure is related to the diffusion barrier function is unclear. Here, we performed single-particle tracking time-course experiments on hippocampal neurons during AIS development. We analyzed the mobility of lipid-anchored molecules by high-speed single-particle tracking and correlated positions of membrane molecules with the nanoscopic organization of the AIS cytoskeleton. We observe a strong reduction in mobility early in AIS development. Membrane protein motion in the AIS plasma membrane is confined to a repetitive pattern of ∼190-nm-spaced segments along the AIS axis as early as day in vitro 4, and this pattern alternates with actin rings. Mathematical modeling shows that diffusion barriers between the segments significantly reduce lateral diffusion along the axon.
© 2016 Albrecht et al.
Figures




References
-
- Andrade D.M., Clausen M.P., Keller J., Mueller V., Wu C., Bear J.E., Hell S.W., Lagerholm B.C., and Eggeling C.. 2015. Cortical actin networks induce spatio-temporal confinement of phospholipids in the plasma membrane: a minimally invasive investigation by STED-FCS. Sci. Rep. 5:11454 10.1038/srep11454 - DOI - PMC - PubMed
-
- Boiko T., Vakulenko M., Ewers H., Yap C.C., Norden C., and Winckler B.. 2007. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J. Neurosci. 27:590–603. 10.1523/JNEUROSCI.4302-06.2007 - DOI - PMC - PubMed
-
- Brachet A., Leterrier C., Irondelle M., Fache M.-P., Racine V., Sibarita J.-B., Choquet D., and Dargent B.. 2010. Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. J. Cell Biol. 191:383–395. 10.1083/jcb.201003042 - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

