Cytoskeletal Integrators: The Spectrin Superfamily
- PMID: 27698030
- PMCID: PMC5046693
- DOI: 10.1101/cshperspect.a018259
Cytoskeletal Integrators: The Spectrin Superfamily
Abstract
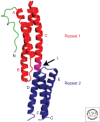
This review discusses the spectrin superfamily of proteins that function to connect cytoskeletal elements to each other, the cell membrane, and the nucleus. The signature domain is the spectrin repeat, a 106-122-amino-acid segment comprising three α-helices. α-actinin is considered to be the ancestral protein and functions to cross-link actin filaments. It then evolved to generate spectrin and dystrophin that function to link the actin cytoskeleton to the cell membrane, as well as the spectraplakins and plakins that link cytoskeletal elements to each other and to junctional complexes. A final class comprises the nesprins, which are able to bind to the nuclear membrane. This review discusses the domain organization of the various spectrin family members, their roles in protein-protein interactions, and their roles in disease, as determined from mutations, and it also describes the functional roles of the family members as determined from null phenotypes.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
