Aag Hypoxanthine-DNA Glycosylase Is Synthesized in the Forespore Compartment and Involved in Counteracting the Genotoxic and Mutagenic Effects of Hypoxanthine and Alkylated Bases in DNA during Bacillus subtilis Sporulation
- PMID: 27698084
- PMCID: PMC5116938
- DOI: 10.1128/JB.00625-16
Aag Hypoxanthine-DNA Glycosylase Is Synthesized in the Forespore Compartment and Involved in Counteracting the Genotoxic and Mutagenic Effects of Hypoxanthine and Alkylated Bases in DNA during Bacillus subtilis Sporulation
Abstract
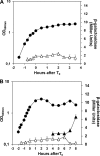
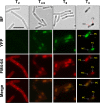
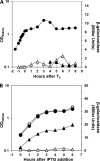
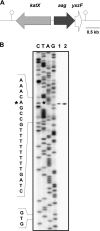
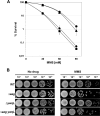
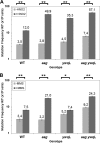
Aag from Bacillus subtilis has been implicated in in vitro removal of hypoxanthine and alkylated bases from DNA. The regulation of expression of aag in B. subtilis and the resistance to genotoxic agents and mutagenic properties of an Aag-deficient strain were studied here. A strain with a transcriptional aag-lacZ fusion expressed low levels of β-galactosidase during growth and early sporulation but exhibited increased transcription during late stages of this developmental process. Notably, aag-lacZ expression was higher inside the forespore than in the mother cell compartment, and this expression was abolished in a sigG-deficient background, suggesting a forespore-specific mechanism of aag transcription. Two additional findings supported this suggestion: (i) expression of an aag-yfp fusion was observed in the forespore, and (ii) in vivo mapping of the aag transcription start site revealed the existence of upstream regulatory sequences possessing homology to σG-dependent promoters. In comparison with the wild-type strain, disruption of aag significantly reduced survival of sporulating B. subtilis cells following nitrous acid or methyl methanesulfonate treatments, and the Rifr mutation frequency was significantly increased in an aag strain. These results suggest that Aag protects the genome of developing B. subtilis sporangia from the cytotoxic and genotoxic effects of base deamination and alkylation.
Importance: In this study, evidence is presented revealing that aag, encoding a DNA glycosylase implicated in processing of hypoxanthine and alkylated DNA bases, exhibits a forespore-specific pattern of gene expression during B. subtilis sporulation. Consistent with this spatiotemporal mode of expression, Aag was found to protect the sporulating cells of this microorganism from the noxious and mutagenic effects of base deamination and alkylation.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. 2006. DNA repair and mutagenesis, 2nd ed American Society for Microbiology, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

