Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus
- PMID: 27698132
- PMCID: PMC5081650
- DOI: 10.1073/pnas.1609316113
Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus
Abstract
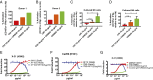
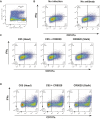
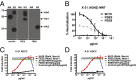
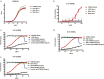
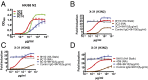
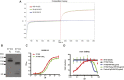
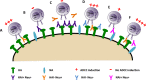
The generation of strain-specific neutralizing antibodies against influenza A virus is known to confer potent protection against homologous infections. The majority of these antibodies bind to the hemagglutinin (HA) head domain and function by blocking the receptor binding site, preventing infection of host cells. Recently, elicitation of broadly neutralizing antibodies which target the conserved HA stalk domain has become a promising "universal" influenza virus vaccine strategy. The ability of these antibodies to elicit Fc-dependent effector functions has emerged as an important mechanism through which protection is achieved in vivo. However, the way in which Fc-dependent effector functions are regulated by polyclonal influenza virus-binding antibody mixtures in vivo has never been defined. Here, we demonstrate that interactions among viral glycoprotein-binding antibodies of varying specificities regulate the magnitude of antibody-dependent cell-mediated cytotoxicity induction. We show that the mechanism responsible for this phenotype relies upon competition for binding to HA on the surface of infected cells and virus particles. Nonneutralizing antibodies were poor inducers and did not inhibit antibody-dependent cell-mediated cytotoxicity. Interestingly, anti-neuraminidase antibodies weakly induced antibody-dependent cell-mediated cytotoxicity and enhanced induction in the presence of HA stalk-binding antibodies in an additive manner. Our data demonstrate that antibody specificity plays an important role in the regulation of ADCC, and that cross-talk among antibodies of varying specificities determines the magnitude of Fc receptor-mediated effector functions.
Keywords: ADCC; Fc receptor; NK cell; antibody; influenza virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine.Front Immunol. 2019 Mar 18;10:440. doi: 10.3389/fimmu.2019.00440. eCollection 2019. Front Immunol. 2019. PMID: 30949165 Free PMC article. Review.
-
Divergent Requirement of Fc-Fcγ Receptor Interactions for In Vivo Protection against Influenza Viruses by Two Pan-H5 Hemagglutinin Antibodies.J Virol. 2017 May 12;91(11):e02065-16. doi: 10.1128/JVI.02065-16. Print 2017 Jun 1. J Virol. 2017. PMID: 28331095 Free PMC article.
-
Dissection of Epitope-Specific Mechanisms of Neutralization of Influenza Virus by Intact IgG and Fab Fragments.J Virol. 2018 Feb 26;92(6):e02006-17. doi: 10.1128/JVI.02006-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263254 Free PMC article.
-
Broadly Cross-Reactive, Nonneutralizing Antibodies against Influenza B Virus Hemagglutinin Demonstrate Effector Function-Dependent Protection against Lethal Viral Challenge in Mice.J Virol. 2019 Mar 5;93(6):e01696-18. doi: 10.1128/JVI.01696-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626682 Free PMC article.
-
Influenza A Virus Antibodies with Antibody-Dependent Cellular Cytotoxicity Function.Viruses. 2020 Mar 1;12(3):276. doi: 10.3390/v12030276. Viruses. 2020. PMID: 32121563 Free PMC article. Review.
Cited by
-
A cross-reactive mouse monoclonal antibody against rhinovirus mediates phagocytosis in vitro.Sci Rep. 2020 Jun 16;10(1):9750. doi: 10.1038/s41598-020-66600-x. Sci Rep. 2020. PMID: 32546721 Free PMC article.
-
Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine.Front Immunol. 2019 Mar 18;10:440. doi: 10.3389/fimmu.2019.00440. eCollection 2019. Front Immunol. 2019. PMID: 30949165 Free PMC article. Review.
-
Influenza H3 hemagglutinin vaccine with scrambled immunodominant epitopes elicits antibodies directed toward immunosubdominant head epitopes.mBio. 2023 Aug 31;14(4):e0062223. doi: 10.1128/mbio.00622-23. Epub 2023 Jul 19. mBio. 2023. PMID: 37466314 Free PMC article.
-
Antibody-Dependent Cell-Mediated Cytotoxicity Epitopes on the Hemagglutinin Head Region of Pandemic H1N1 Influenza Virus Play Detrimental Roles in H1N1-Infected Mice.Front Immunol. 2017 Mar 21;8:317. doi: 10.3389/fimmu.2017.00317. eCollection 2017. Front Immunol. 2017. PMID: 28377769 Free PMC article.
-
Antibodies Directed toward Neuraminidase N1 Control Disease in a Mouse Model of Influenza.J Virol. 2018 Jan 30;92(4):e01584-17. doi: 10.1128/JVI.01584-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29167342 Free PMC article.
References
-
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14(3):167–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

