Hierarchical phosphorylation of apical membrane antigen 1 is required for efficient red blood cell invasion by malaria parasites
- PMID: 27698395
- PMCID: PMC5048298
- DOI: 10.1038/srep34479
Hierarchical phosphorylation of apical membrane antigen 1 is required for efficient red blood cell invasion by malaria parasites
Abstract
Central to the pathogenesis of malaria is the proliferation of Plasmodium falciparum parasites within human erythrocytes. Parasites invade erythrocytes via a coordinated sequence of receptor-ligand interactions between the parasite and host cell. One key ligand, Apical Membrane Antigen 1 (AMA1), is a leading blood-stage vaccine and previous work indicates that phosphorylation of its cytoplasmic domain (CPD) is important to its function during invasion. Here we investigate the significance of each of the six available phospho-sites in the CPD. We confirm that the cyclic AMP/protein kinase A (PKA) signalling pathway elicits a phospho-priming step upon serine 610 (S610), which enables subsequent phosphorylation in vitro of a conserved, downstream threonine residue (T613) by glycogen synthase kinase 3 (GSK3). Both phosphorylation steps are required for AMA1 to function efficiently during invasion. This provides the first evidence that the functions of key invasion ligands of the malaria parasite are regulated by sequential phosphorylation steps.
Figures
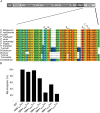
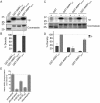
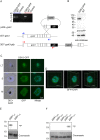
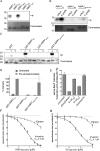
References
-
- World Health Organization. World Malaria Report 2014 (World Health Organization, 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

