Predicting the functional states of human iPSC-derived neurons with single-cell RNA-seq and electrophysiology
- PMID: 27698428
- PMCID: PMC5071135
- DOI: 10.1038/mp.2016.158
Predicting the functional states of human iPSC-derived neurons with single-cell RNA-seq and electrophysiology
Abstract
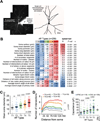
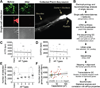
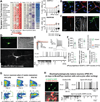
Human neural progenitors derived from pluripotent stem cells develop into electrophysiologically active neurons at heterogeneous rates, which can confound disease-relevant discoveries in neurology and psychiatry. By combining patch clamping, morphological and transcriptome analysis on single-human neurons in vitro, we defined a continuum of poor to highly functional electrophysiological states of differentiated neurons. The strong correlations between action potentials, synaptic activity, dendritic complexity and gene expression highlight the importance of methods for isolating functionally comparable neurons for in vitro investigations of brain disorders. Although whole-cell electrophysiology is the gold standard for functional evaluation, it often lacks the scalability required for disease modeling studies. Here, we demonstrate a multimodal machine-learning strategy to identify new molecular features that predict the physiological states of single neurons, independently of the time spent in vitro. As further proof of concept, we selected one of the potential neurophysiological biomarkers identified in this study-GDAP1L1-to isolate highly functional live human neurons in vitro.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. - PubMed
-
- Cho M-S, Hwang D-Y, Kim D-W. Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc. 2008;3:1888–1894. - PubMed
-
- Vadodaria KC, Mertens J, Paquola A, Bardy C, Li X, Jappelli R, et al. Generation of functional human serotonergic neurons from fibroblasts. Molecular Psychiatry. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

