Deciphering KRAS and NRAS mutated clone dynamics in MLL-AF4 paediatric leukaemia by ultra deep sequencing analysis
- PMID: 27698462
- PMCID: PMC5048141
- DOI: 10.1038/srep34449
Deciphering KRAS and NRAS mutated clone dynamics in MLL-AF4 paediatric leukaemia by ultra deep sequencing analysis
Abstract
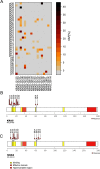
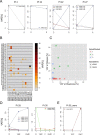
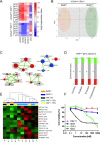
To induce and sustain the leukaemogenic process, MLL-AF4+ leukaemia seems to require very few genetic alterations in addition to the fusion gene itself. Studies of infant and paediatric patients with MLL-AF4+ B cell precursor acute lymphoblastic leukaemia (BCP-ALL) have reported mutations in KRAS and NRAS with incidences ranging from 25 to 50%. Whereas previous studies employed Sanger sequencing, here we used next generation amplicon deep sequencing for in depth evaluation of RAS mutations in 36 paediatric patients at diagnosis of MLL-AF4+ leukaemia. RAS mutations including those in small sub-clones were detected in 63.9% of patients. Furthermore, the mutational analysis of 17 paired samples at diagnosis and relapse revealed complex RAS clone dynamics and showed that the mutated clones present at relapse were almost all originated from clones that were already detectable at diagnosis and survived to the initial therapy. Finally, we showed that mutated patients were indeed characterized by a RAS related signature at both transcriptional and protein levels and that the targeting of the RAS pathway could be of beneficial for treatment of MLL-AF4+ BCP-ALL clones carrying somatic RAS mutations.
Figures
Similar articles
-
Inhibition of MEK and ATR is effective in a B-cell acute lymphoblastic leukemia model driven by Mll-Af4 and activated Ras.Blood Adv. 2018 Oct 9;2(19):2478-2490. doi: 10.1182/bloodadvances.2018021592. Blood Adv. 2018. PMID: 30266823 Free PMC article.
-
Analysis of KRAS and NRAS Gene Mutations in Arab Asian Children With Acute Leukemia: High Frequency of RAS Mutations in Acute Lymphoblastic Leukemia.Pediatr Blood Cancer. 2015 Dec;62(12):2157-61. doi: 10.1002/pbc.25683. Epub 2015 Jul 29. Pediatr Blood Cancer. 2015. PMID: 26222068 Clinical Trial.
-
Mutational status of NRAS, KRAS, and PTPN11 genes is associated with genetic/cytogenetic features in children with B-precursor acute lymphoblastic leukemia.Pediatr Blood Cancer. 2018 Feb;65(2). doi: 10.1002/pbc.26786. Epub 2017 Aug 29. Pediatr Blood Cancer. 2018. PMID: 28853218 Clinical Trial.
-
Insights into the cellular origin and etiology of the infant pro-B acute lymphoblastic leukemia with MLL-AF4 rearrangement.Leukemia. 2011 Mar;25(3):400-10. doi: 10.1038/leu.2010.284. Epub 2010 Dec 7. Leukemia. 2011. PMID: 21135858 Review.
-
Structural fingerprints, interactions, and signaling networks of RAS family proteins beyond RAS isoforms.Crit Rev Biochem Mol Biol. 2018 Apr;53(2):130-156. doi: 10.1080/10409238.2018.1431605. Epub 2018 Feb 19. Crit Rev Biochem Mol Biol. 2018. PMID: 29457927 Review. No abstract available.
Cited by
-
Updates in KMT2A Gene Rearrangement in Pediatric Acute Lymphoblastic Leukemia.Biomedicines. 2023 Mar 8;11(3):821. doi: 10.3390/biomedicines11030821. Biomedicines. 2023. PMID: 36979800 Free PMC article. Review.
-
Blinatumomab-induced lineage switch of B-ALL with t(4:11)(q21;q23) KMT2A/AFF1 into an aggressive AML: pre- and post-switch phenotypic, cytogenetic and molecular analysis.Blood Cancer J. 2017 Sep 15;7(9):e607. doi: 10.1038/bcj.2017.89. Blood Cancer J. 2017. PMID: 29016570 Free PMC article. No abstract available.
-
Clinical profile in KMT2A-SEPT6-positive acute myeloid leukemia: Does it often co-occur with NRAS mutations?Front Med (Lausanne). 2022 Sep 21;9:890959. doi: 10.3389/fmed.2022.890959. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36213638 Free PMC article.
-
The Impact of PI3-kinase/RAS Pathway Cooperating Mutations in the Evolution of KMT2A-rearranged Leukemia.Hemasphere. 2019 Jun 4;3(3):e195. doi: 10.1097/HS9.0000000000000195. eCollection 2019 Jun. Hemasphere. 2019. PMID: 31723831 Free PMC article. Review.
-
Inhibition of MEK and ATR is effective in a B-cell acute lymphoblastic leukemia model driven by Mll-Af4 and activated Ras.Blood Adv. 2018 Oct 9;2(19):2478-2490. doi: 10.1182/bloodadvances.2018021592. Blood Adv. 2018. PMID: 30266823 Free PMC article.
References
-
- Pui C. H. et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia 17, 700–706 (2003). - PubMed
-
- Mann G. et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood 116, 2644–2650 (2010). - PubMed
-
- Ayton P. M. & Cleary M. L. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene 20, 5695–5707 (2001). - PubMed
-
- Krivtsov A. V. & Armstrong S. A. MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer. 7, 823–833 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

