Apocynin and Nox2 regulate NF-κB by modifying thioredoxin-1 redox-state
- PMID: 27698473
- PMCID: PMC5048297
- DOI: 10.1038/srep34581
Apocynin and Nox2 regulate NF-κB by modifying thioredoxin-1 redox-state
Abstract
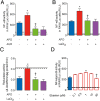
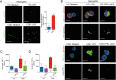
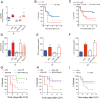
The reactive-oxygen-species-(ROS)-generating-enzyme Nox2 is essential for leukocyte anti-microbial activity. However its role in cellular redox homeostasis and, consequently, in modulating intracellular signaling pathways remains unclear. Herein, we show Nox2 activation favors thioredoxin-1 (TRX-1)/p40phox interaction, which leads to exclusion of TRX-1 from the nucleus. In contrast, the genetic deficiency of Nox2 or its pharmacological inhibition with apocynin (APO) results in reductive stress after lipopolysaccharide-(LPS)-cell stimulation, which causes nuclear accumulation of TRX-1 and enhanced transcription of inflammatory mediators through nuclear-factor-(NF)-κB. The NF-κB overactivation is prevented by TRX-1 oxidation using inhibitors of thioredoxin reductase-1 (TrxR-1). The Nox2/TRX-1/NF-κB intracellular signaling pathway is involved in the pathophysiology of chronic granulomatous disease (CGD) and sepsis. In fact, TrxR-1 inhibition prevents nuclear accumulation of TRX-1 and LPS-stimulated hyperproduction of tumor-necrosis-factor-(TNF)-α by monocytes and neutrophils purified from blood of CGD patients, who have deficient Nox2 activity. TrxR-1 inhibitors, either lanthanum chloride (LaCl3) or auranofin (AUR), also increase survival rates of mice undergoing cecal-ligation-and-puncture-(CLP). Therefore, our results identify a hitherto unrecognized Nox2-mediated intracellular signaling pathway that contributes to hyperinflammation in CGD and in septic patients. Additionally, we suggest that TrxR-1 inhibitors could be potential drugs to treat patients with sepsis, particularly in those with CGD.
Figures




References
-
- Dinauer M. C. & Orkin S. H. Chronic granulomatous disease. Annu Rev Med. 43, 117–124 (1992). - PubMed
-
- Pollock J. D. et al.. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 9, 202–209 (1995). - PubMed
-
- Deffert C. et al.. Hyperinflammation of chronic granulomatous disease is abolished by Nox2 reconstitution in macrophages and dendritic cells. J Pathol. 228, 341–350 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

