Synthesis of Psoralidin derivatives and their anticancer activity: First synthesis of Lespeflorin I1
- PMID: 27698514
- PMCID: PMC5044874
- DOI: 10.1016/j.tet.2016.04.066
Synthesis of Psoralidin derivatives and their anticancer activity: First synthesis of Lespeflorin I1
Abstract
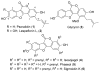
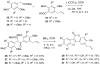
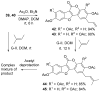
Synthetic scheme for the preparation of a number of different derivatives of anticancer natural product Psoralidin is described. A convergent synthetic approach is followed using simple starting materials like substituted phenyl acetic esters and benzoic acids. The developed synthetic route leads us to complete the first synthesis of an analogous natural product Lespeflorin I1, a mild melanin synthesis inhibitor. Preliminary bioactivity studies of the synthesized compounds are carried out against two commonly used prostate cancer cell lines. Results show that the bioactivity of the compounds can be manipulated by the simple modification of the functional groups.
Figures


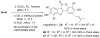
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
