Esomeprazole- or rabeprazole-based triple therapy eradicated Helicobacter pylori comparably regardless of clarithromycin susceptibility and CYP2C19 genotypes
- PMID: 27698544
- PMCID: PMC5018575
- DOI: 10.3164/jcbn.16-18
Esomeprazole- or rabeprazole-based triple therapy eradicated Helicobacter pylori comparably regardless of clarithromycin susceptibility and CYP2C19 genotypes
Abstract
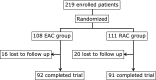
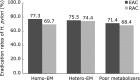
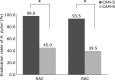
The aim of this study was to assess the efficacy of esomeprazole-based triple therapy compared with rabeprazole-based triple therapy according to CYP2C19 genotype and clarithromycin susceptibility status for first-line eradication therapy of Helicobacter pylori (H. pylori) in Japan. We enrolled 219 H. pylori-infected patients, and randomly allocated patients to the EAC group (esomeprazole 20 mg, clarithromycin 200 mg, amoxicillin 750 mg for one week, with all drugs given twice daily) or RAC group (rabeprazole 10 mg, clarithromycin 200 mg, amoxicillin 750 mg for one week, with all drugs given twice daily). The H. pylori eradication rate according to the PP analyses was 75.0% (95% CI: 65.2-82.8%) in the EAC group and 71.4% (95% CI: 61.4-79.1%) in the RAC group. There were no statistically significant differences. The eradication rates of the clarithromycin-resistant/-sensitive strains were, respectively, 45.0% (95% CI: 30.7-60.2%)/98.0% (95% CI: 88.7-100%) in the EAC group and 39.5% (95% CI: 25.6-55.3%)/93.5% (95% CI: 81.9-98.4%) in the RAC group. The eradication rate of the clarithromycin-sensitive strains was significantly higher than that of the resistant strains in both groups. In conclusion, EAC and RAC therapies show a comparable efficacy regardless of the CYP2C19 genotype and clarithromycin susceptibility status in Japan.
Keywords: CYP2C19; Helicobacter pylori; eradication; esomeprazole; rabeprazole.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Randomized Trial Comparing Esomeprazole and Rabeprazole in First-line Eradication Therapy for Helicobacter pylori Infection based on the Serum Levels of Pepsinogens.Intern Med. 2017;56(13):1621-1627. doi: 10.2169/internalmedicine.56.7823. Epub 2017 Jul 1. Intern Med. 2017. PMID: 28674348 Free PMC article. Clinical Trial.
-
Effects of CYP2C19 gene polymorphism on cure rates for Helicobacter pylori infection by triple therapy with proton pump inhibitor (omeprazole or rabeprazole), amoxycillin and clarithromycin in Japan.Dig Liver Dis. 2001 Nov;33(8):671-5. doi: 10.1016/s1590-8658(01)80043-8. Dig Liver Dis. 2001. PMID: 11785712 Clinical Trial.
-
Effect of different proton pump inhibitors, differences in CYP2C19 genotype and antibiotic resistance on the eradication rate of Helicobacter pylori infection by a 1-week regimen of proton pump inhibitor, amoxicillin and clarithromycin.Aliment Pharmacol Ther. 2003 Jan;17(2):259-64. doi: 10.1046/j.1365-2036.2003.01406.x. Aliment Pharmacol Ther. 2003. PMID: 12534411 Clinical Trial.
-
Influence of Cytochrome P450 2C19 Genotype on Helicobacter pylori Proton Pump Inhibitor-Amoxicillin-Clarithromycin Eradication Therapy: A Meta-Analysis.Front Pharmacol. 2021 Oct 15;12:759249. doi: 10.3389/fphar.2021.759249. eCollection 2021. Front Pharmacol. 2021. PMID: 34721043 Free PMC article. Review.
-
Standard triple therapy in Helicobacter pylori eradication in Turkey: Systematic evaluation and meta-analysis of 10-year studies.Turk J Gastroenterol. 2019 May;30(5):420-435. doi: 10.5152/tjg.2019.18693. Turk J Gastroenterol. 2019. PMID: 31060997 Free PMC article.
Cited by
-
A Vonoprazan, Clarithromycin, and Metronidazole Regimen as Helicobacter pylori Eradication Therapy for Patients with Penicillin Allergy in Light of Clarithromycin Resistance.Intern Med. 2023 Aug 15;62(16):2301-2306. doi: 10.2169/internalmedicine.0789-22. Epub 2023 Jan 12. Intern Med. 2023. PMID: 36631092 Free PMC article.
-
Effect of MDR1 C3435T and CYP2C19 genetic polymorphisms on the outcome of Helicobacter pylori eradication treatment in children with gastritis and peptic ulcer, Vietnam.BMC Pediatr. 2024 Jul 19;24(1):464. doi: 10.1186/s12887-024-04581-w. BMC Pediatr. 2024. PMID: 39030549 Free PMC article.
References
-
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. - PubMed
-
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. - PubMed
-
- Kawai T, Takahashi S, Suzuki H, et al. Changes in the first line Helicobacter pylori eradication rates using the triple therapy-a multicenter study in the Tokyo metropolitan area (Tokyo Helicobacter pylori study group) J Gastroenterol Hepatol. 2014;29 Suppl 4:29–32. - PubMed
-
- Calvet X, Gomollón F. What is potent acid inhibition, and how can it be achieved? Drugs. 2005;65 Suppl 1:13–23. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

