Protective Effect of Galectin-1 during Histoplasma capsulatum Infection Is Associated with Prostaglandin E2 and Nitric Oxide Modulation
- PMID: 27698545
- PMCID: PMC5028869
- DOI: 10.1155/2016/5813794
Protective Effect of Galectin-1 during Histoplasma capsulatum Infection Is Associated with Prostaglandin E2 and Nitric Oxide Modulation
Abstract
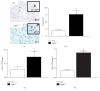
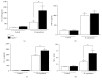
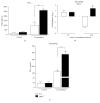
Histoplasma capsulatum is a dimorphic fungus that develops a yeast-like morphology in host's tissue, responsible for the pulmonary disease histoplasmosis. The recent increase in the incidence of histoplasmosis in immunocompromised patients highlights the need of understanding immunological controls of fungal infections. Here, we describe our discovery of the role of endogenous galectin-1 (Gal-1) in the immune pathophysiology of experimental histoplasmosis. All infected wild-type (WT) mice survived while only 1/3 of Lgals1-/- mice genetically deficient in Gal-1 survived 30 days after infection. Although infected Lgals1-/- mice had increased proinflammatory cytokines, nitric oxide (NO), and elevations in neutrophil pulmonary infiltration, they presented higher fungal load in lungs and spleen. Infected lung and infected macrophages from Lgals1-/- mice exhibited elevated levels of prostaglandin E2 (PGE2, a prostanoid regulator of macrophage activation) and prostaglandin E synthase 2 (Ptgs2) mRNA. Gal-1 did not bind to cell surface of yeast phase of H. capsulatum, in vitro, suggesting that Gal-1 contributed to phagocytes response to infection rather than directly killing the yeast. The data provides the first demonstration of endogenous Gal-1 in the protective immune response against H. capsulatum associated with NO and PGE2 as an important lipid mediator in the pathogenesis of histoplasmosis.
Figures



Similar articles
-
Biodiverse Histoplasma Species Elicit Distinct Patterns of Pulmonary Inflammation following Sublethal Infection.mSphere. 2020 Aug 26;5(4):e00742-20. doi: 10.1128/mSphere.00742-20. mSphere. 2020. PMID: 32848006 Free PMC article.
-
Gr-1+ cells play an essential role in an experimental model of disseminated histoplasmosis.Microbes Infect. 2007 Oct;9(12-13):1393-401. doi: 10.1016/j.micinf.2006.10.007. Epub 2006 Dec 12. Microbes Infect. 2007. PMID: 17296322
-
Regulation of infection with Histoplasma capsulatum by TNFR1 and -2.J Immunol. 2000 Sep 1;165(5):2657-64. doi: 10.4049/jimmunol.165.5.2657. J Immunol. 2000. PMID: 10946295
-
Usefulness of the murine model to study the immune response against Histoplasma capsulatum infection.Comp Immunol Microbiol Infect Dis. 2014 May;37(3):143-52. doi: 10.1016/j.cimid.2014.03.002. Epub 2014 Apr 6. Comp Immunol Microbiol Infect Dis. 2014. PMID: 24766724 Review.
-
Histoplasma capsulatum molecular genetics, pathogenesis, and responsiveness to its environment.Fungal Genet Biol. 2002 Mar;35(2):81-97. doi: 10.1006/fgbi.2001.1311. Fungal Genet Biol. 2002. PMID: 11848673 Review.
Cited by
-
The role of galectins in immunity and infection.Nat Rev Immunol. 2023 Aug;23(8):479-494. doi: 10.1038/s41577-022-00829-7. Epub 2023 Jan 16. Nat Rev Immunol. 2023. PMID: 36646848 Free PMC article. Review.
-
The Role of Collectins and Galectins in Lung Innate Immune Defense.Front Immunol. 2018 Sep 4;9:1998. doi: 10.3389/fimmu.2018.01998. eCollection 2018. Front Immunol. 2018. PMID: 30233589 Free PMC article. Review.
-
Galectin-1 mediates interactions between polymorphonuclear leukocytes and vascular endothelial cells, and promotes their extravasation during lipopolysaccharide-induced acute lung injury.Mol Immunol. 2023 Apr;156:127-135. doi: 10.1016/j.molimm.2023.02.011. Epub 2023 Mar 13. Mol Immunol. 2023. PMID: 36921487 Free PMC article.
-
The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function.Front Immunol. 2019 Aug 7;10:1762. doi: 10.3389/fimmu.2019.01762. eCollection 2019. Front Immunol. 2019. PMID: 31440233 Free PMC article. Review.
-
Complex and Controversial Roles of Eicosanoids in Fungal Pathogenesis.J Fungi (Basel). 2021 Mar 28;7(4):254. doi: 10.3390/jof7040254. J Fungi (Basel). 2021. PMID: 33800694 Free PMC article. Review.
References
-
- Allendoerfer R., Deepe G. S., Jr. Infection with Histoplasma capsulatum: host-fungus interface. Revista Iberoamericana de Micologia. 1998;15(4):256–260. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

