Unraveling the Dynamics of the Human Vaginal Microbiome
- PMID: 27698617
- PMCID: PMC5045142
Unraveling the Dynamics of the Human Vaginal Microbiome
Abstract
Four Lactobacillus species, namely L. crispatus , L. iners, L. gasseri, and L. jensenii, commonly dominate the vaginal communities of most reproductive-age women. It is unclear why these particular species, and not others, are so prevalent. Historically, estrogen-induced glycogen production by the vaginal epithelium has been proffered as being key to supporting the proliferation of vaginal lactobacilli. However, the 'fly in the ointment' (that has been largely ignored) is that the species of Lactobacillus commonly found in the human vagina cannot directly metabolize glycogen. It would appear that this riddle has been solved as studies have demonstrated that vaginal lactobacilli can metabolize the products of glycogen depolymerization by α-amylase, and fortunately, amylase activity is found in vaginal secretions. These amylases are presumed to be host-derived, but we suggest that other bacterial populations in vaginal communities could also be sources of amylase in addition to (or instead of) the host. Here we briefly review what is known about human vaginal bacterial communities and discuss how glycogen-derived resources and resource competition might shape the composition and structure of these communities.
Keywords: alpha-amylase; glycogen; microbial community; microbiome; vagina; vaginal microbiome.
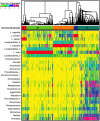
Figures


References
-
- Zhou X, Bent SJ, Schneider MG. et al. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(Pt 8):2565–2573. - PubMed
-
- Zhou X, Brown CJ, Abdo Z. et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. The ISME Journal. 2007;1(2):121–133. - PubMed
-
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infec Dis. 1999;180(6):1950–1956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
