Homocysteine injures vascular endothelial cells by inhibiting mitochondrial activity
- PMID: 27698720
- PMCID: PMC5038564
- DOI: 10.3892/etm.2016.3564
Homocysteine injures vascular endothelial cells by inhibiting mitochondrial activity
Abstract
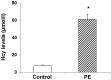
The aim of the present study was to investigate the role of homocysteine (Hcy) in the pathogenesis of pulmonary embolism (PE) and the associated molecular mechanisms in human umbilical vein endothelial cells (HUVECs). Hcy contents were detected with high-performance liquid chromatography. Apoptosis was detected by flow cytometry using Annexin-V staining. Cytochrome c oxidase (COX) activity was assessed with an enzyme activity assay, and the expression levels of COX 17 were determined by western blot analysis. Intracellular reactive oxygen species levels were measured using a microplate reader with a fluorescence probe. The results demonstrated that, compared with the control group, the serum Hcy levels were significantly elevated in the PE group, suggesting that Hcy may be an indicator for PE. Following treatment with Hcy, the apoptosis rate was markedly elevated in HUVECs. Moreover, Hcy decreased COX activity and downregulated the expression of COX 17 in HUVECs. Furthermore, Hcy increased the ROS levels in these endothelial cells. However, all the above-mentioned physiopathological changes induced by Hcy in HUVECs could be restored by folic acid. In conclusion, the results of the present study demonstrated that Hcy inhibited COX activity, downregulated COX 17 expression, increased intracellular ROS levels and enhanced apoptosis in endothelial cells.
Keywords: apoptosis; cytochrome c oxidase; homocysteine; pulmonary embolism; vascular endothelial cells.
Figures


Similar articles
-
Melatonin attenuates homocysteine-induced injury in human umbilical vein endothelial cells.Fundam Clin Pharmacol. 2018 Jun;32(3):261-269. doi: 10.1111/fcp.12355. Epub 2018 Mar 24. Fundam Clin Pharmacol. 2018. PMID: 29436019
-
HSP27 Inhibits Homocysteine-Induced Endothelial Apoptosis by Modulation of ROS Production and Mitochondrial Caspase-Dependent Apoptotic Pathway.Biomed Res Int. 2016;2016:4847874. doi: 10.1155/2016/4847874. Epub 2016 Apr 17. Biomed Res Int. 2016. PMID: 27190988 Free PMC article.
-
Protective effects of oxymatrine on homocysteine-induced endothelial injury: Involvement of mitochondria-dependent apoptosis and Akt-eNOS-NO signaling pathways.Eur J Pharmacol. 2019 Dec 1;864:172717. doi: 10.1016/j.ejphar.2019.172717. Epub 2019 Oct 3. Eur J Pharmacol. 2019. PMID: 31586637
-
S-Adenosylhomocysteine induces apoptosis and phosphatidylserine exposure in endothelial cells independent of homocysteine.Atherosclerosis. 2012 Mar;221(1):48-54. doi: 10.1016/j.atherosclerosis.2011.11.032. Epub 2011 Nov 28. Atherosclerosis. 2012. PMID: 22204864
-
Homocysteine induced oxidative stress in human umbilical vein endothelial cells via regulating methylation of SORBS1.Eur Rev Med Pharmacol Sci. 2018 Oct;22(20):6948-6958. doi: 10.26355/eurrev_201810_16164. Eur Rev Med Pharmacol Sci. 2018. PMID: 30402861
Cited by
-
Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems.Int J Mol Sci. 2020 Oct 18;21(20):7698. doi: 10.3390/ijms21207698. Int J Mol Sci. 2020. PMID: 33080955 Free PMC article. Review.
-
Photobiomodulation improves depression symptoms: a systematic review and meta-analysis of randomized controlled trials.Front Psychiatry. 2024 Jan 31;14:1267415. doi: 10.3389/fpsyt.2023.1267415. eCollection 2023. Front Psychiatry. 2024. PMID: 38356614 Free PMC article.
-
Oxidative stress in acute pulmonary embolism: emerging roles and therapeutic implications.Thromb J. 2024 Jan 12;22(1):9. doi: 10.1186/s12959-023-00577-1. Thromb J. 2024. PMID: 38216919 Free PMC article. Review.
-
Therapeutic effect of folic acid combined with decitabine on diabetic mice.Int J Ophthalmol. 2023 Nov 18;16(11):1766-1772. doi: 10.18240/ijo.2023.11.05. eCollection 2023. Int J Ophthalmol. 2023. PMID: 38028519 Free PMC article.
-
Endothelial Cell Metabolism.Physiol Rev. 2018 Jan 1;98(1):3-58. doi: 10.1152/physrev.00001.2017. Physiol Rev. 2018. PMID: 29167330 Free PMC article. Review.
References
-
- de Miguel-Díez J, Jiménez-García R, de Andrés A López, Hernández-Barrera V, Carrasco-Garrido P, Monreal M, Jiménez D, Jara-Palomares L, Álvaro-Meca A. Analysis of environmental risk factors for pulmonary embolism: A case-crossover study (2001-2013) Eur J Intern Med. 2016;31:55–61. doi: 10.1016/j.ejim.2016.03.001. - DOI - PubMed
-
- Chen P, Poddar R, Tipa EV, Dibello PM, Moravec CD, Robinson K, Green R, Kruger WD, Garrow TA, Jacobsen DW. Homocysteine metabolism in cardiovascular cells and tissues: Implications for hyperhomocysteinemia and cardiovascular disease. Adv Enzyme Regul. 1999;39:93–109. doi: 10.1016/S0065-2571(98)00029-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
