Recombinant expression and biological characterization of the antimicrobial peptide fowlicidin-2 in Pichia pastoris
- PMID: 27698732
- PMCID: PMC5038210
- DOI: 10.3892/etm.2016.3578
Recombinant expression and biological characterization of the antimicrobial peptide fowlicidin-2 in Pichia pastoris
Abstract
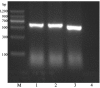
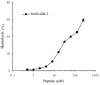
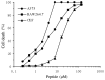
Fowlicidins are a group of cathelicidin antimicrobial peptides that were initially identified in chickens. Fowlicidin-2, which is composed of 31 amino acids, is widely expressed in the majority of tissues in chickens and has an important role in innate immunity. In the present study, a recombinant expression system for fowlicidin-2 was successfully constructed using Pichia pastoris X-33 and the expression vector pPICZα-A. Under the optimized fermentation conditions, 85.6 mg fowlicidin-2 with >95% purity was obtained from 1 liter culture medium following purification by ion exchange chromatography and reversed phase high performance liquid chromatography. The recombinant fowlicidin-2 exhibited broad spectrum antimicrobial activity and had a minimum inhibitory concentration ranging from 1 to 4 µM. Furthermore, recombinant fowlicidin-2 exhibited hemolytic activity, promoting 50% human erythrocyte hemolysis in the concentration range of 128-256 µM, and anticancer activity, resulting in the death of 50% of A375 human malignant melanoma cells in the concentration range of 2-4 µM. The results of the present study suggest that recombinant fowlicidin-2 may be a promising candidate for therapeutic applications.
Keywords: Pichia pastoris; antibacterial activity; anticancer activity; antimicrobial peptides; fowlicidin-2; recombinant expression.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
