Randomized, double-blind, placebo-controlled superiority trial of the Yiqigubiao pill for the treatment of patients with chronic obstructive pulmonary disease at a stable stage
- PMID: 27698749
- PMCID: PMC5038223
- DOI: 10.3892/etm.2016.3680
Randomized, double-blind, placebo-controlled superiority trial of the Yiqigubiao pill for the treatment of patients with chronic obstructive pulmonary disease at a stable stage
Abstract
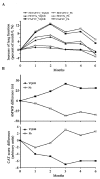
In traditional Chinese medicine (TCM), the Yiqigubiao pill is commonly used to enhance physical fitness. The current clinical trial was designed to evaluate the efficacy and safety of the Yiqigubiao pill as an adjuvant therapy for patients with stable chronic obstructive pulmonary disease (COPD). The current trial was a randomized, double-blind, placebo-controlled superiority trial. The participants were recruited from outpatients at the Traditional Chinese Medicine Hospital affiliated with Xinjiang Medical University (Ürümqi, China) between February and September 2012. All participants were patients with stable COPD that were randomized to the Yiqigubiao pill (YQGB; n=84) or placebo (Pb; n=87) groups. The occurrences of acute exacerbation (AE) of COPD during the trial were recorded. Lung function value assessments, scoring of life quality and exercise endurance, arterial blood gas analysis and serum inflammatory cytokines level determination were performed prior to and throughout the study. A total of 139 participants completed the intervention and 132 participants completed the study. The interval between the initial intervention and the first AECOPD was greater in the YQGB group compared with the Pb group (P<0.01). The incidence rate of AECOPD was lower in the YQGB group than in the Pb group (P<0.01). Subsequent to the intervention or at the end of the study, the 6-min walking distance difference was longer in the YQGB group compared with the Pb group (P<0.01). The scores reflecting life quality decline became lower in the YQGB group (P<0.01). The serum levels of proinflammatory factors were downregulated to a greater extent in the YQGB group compared with the Pb group. Thus, the Yiqigubiao pill is an efficient and safe adjuvant therapy for the treatment of stable patients with COPD.
Keywords: Yiqigubiao pill; chronic obstructive pulmonary disease; randomized controlled trial; stable stage; traditional Chinese medicine.
Figures
Similar articles
-
Yiqigubiao pill treatment regulates Sirtuin 5 expression and mitochondrial function in chronic obstructive pulmonary disease.J Thorac Dis. 2024 Apr 30;16(4):2326-2340. doi: 10.21037/jtd-23-1115. Epub 2024 Apr 16. J Thorac Dis. 2024. PMID: 38738261 Free PMC article.
-
Efficacy of Yiqigubiao pill on chronic obstructive pulmonary disease in rats with the disease induced by lipopolysaccharide and cigarette-smoke fumigation.J Tradit Chin Med. 2020 Dec;40(6):983-991. doi: 10.19852/j.cnki.jtcm.20201104.001. J Tradit Chin Med. 2020. PMID: 33258350
-
Xuan Bai Cheng Qi formula as an adjuvant treatment of acute exacerbation of chronic obstructive pulmonary disease of the syndrome type phlegm-heat obstructing the lungs: a multicenter, randomized, double-blind, placebo-controlled clinical trial.BMC Complement Altern Med. 2014 Jul 11;14:239. doi: 10.1186/1472-6882-14-239. BMC Complement Altern Med. 2014. PMID: 25014996 Free PMC article. Clinical Trial.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
-
Active mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2018 Oct 10;10(10):CD012290. doi: 10.1002/14651858.CD012290.pub2. Cochrane Database Syst Rev. 2018. PMID: 30306545 Free PMC article.
Cited by
-
Long-Term Effects of TCM Yangqing Kangxian Formula on Bleomycin-Induced Pulmonary Fibrosis in Rats via Regulating Nuclear Factor-κB Signaling.Evid Based Complement Alternat Med. 2017;2017:2089027. doi: 10.1155/2017/2089027. Epub 2017 Dec 7. Evid Based Complement Alternat Med. 2017. PMID: 29387126 Free PMC article.
-
A Randomized Controlled Study of the Yi Qi Gu Biao Pill in the Treatment of Frequent Exacerbator Phenotype in Chronic Obstructive Pulmonary Disease (Lung and Spleen Qi Deficiency Syndrome).Evid Based Complement Alternat Med. 2017;2017:9130804. doi: 10.1155/2017/9130804. Epub 2017 Dec 4. Evid Based Complement Alternat Med. 2017. PMID: 29348777 Free PMC article.
-
Yiqigubiao pill treatment regulates Sirtuin 5 expression and mitochondrial function in chronic obstructive pulmonary disease.J Thorac Dis. 2024 Apr 30;16(4):2326-2340. doi: 10.21037/jtd-23-1115. Epub 2024 Apr 16. J Thorac Dis. 2024. PMID: 38738261 Free PMC article.
-
The Clinical Efficiency and the Mechanism of Sanzi Yangqin Decoction for Chronic Obstructive Pulmonary Disease.Evid Based Complement Alternat Med. 2021 Jun 10;2021:5565562. doi: 10.1155/2021/5565562. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34221077 Free PMC article.
-
Yinyanghuo () and its components for chronic obstructive pulmonary disease: preclinical evidence and possible mechanisms.J Tradit Chin Med. 2023 Apr;43(2):386-396. doi: 10.19852/j.cnki.jtcm.20220617.005. J Tradit Chin Med. 2023. PMID: 36994529 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease, corp-author. http://www.goldcopd.com Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (Revised 2011) - PubMed
-
- Almansa R, Socias L, Andaluz-Ojeda D, Martín-Loeches I, Bobillo F, Blanco J, Rico L, Berezo JÁ, Estella Á, Sanchez-Garcia M, et al. Viral infection is associated with an increased proinflammatory response in chronic obstructive pulmonary disease. Viral Immunol. 2012;25:249–253. doi: 10.1089/vim.2011.0095. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
