Oral paracetamol vs. oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: A randomized controlled trial
- PMID: 27698754
- PMCID: PMC5038853
- DOI: 10.3892/etm.2016.3676
Oral paracetamol vs. oral ibuprofen in the treatment of symptomatic patent ductus arteriosus in premature infants: A randomized controlled trial
Abstract
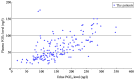
The aim of the present study was to analyze the changes of plasma and urinary prostaglandin E2 (PGE2) levels in preterm infants with symptomatic patent ductus arteriosus (sPDA) treated with oral ibuprofen and acetaminophen. A total of 87 preterm infants with sPDA admitted to the Neonatal Ward of the Affiliated Xuzhou Hospital of Medical College of Southeast University from October, 2012 to June, 2015 were selected and randomly divided into the ibuprofen group (n=43, 10 mg/kg ibuprofen administered orally as initial dose, followed by 5 mg/kg during the first 24 and 48 h later) and acetaminophen group (n=44, 15 mg/kg acetaminophen administered orally once every 6 h for three days). The levels of plasma and urinary PGE2 in the two groups were estimated before and after treatment. The treatment of sPDA infants with ibuprofen (ibuprofen group) or acetaminophen (acetaminophen group) caused a significant decrease in the plasma and urinary PGE2 levels in comparison with plasma and urinary PGE2 levels before treatment (P<0.05). Furthermore, plasma and urinary PGE2 levels in the acetaminophen group (45.0±36.9 ng/l) were significantly lower than those in the ibuprofen group (73.5±44.8 ng/l, P=0.002). The arterial duct closure rate was similar between the acetaminophen [31 (70.5%)] and ibuprofen groups [33 (76.7%), P=0.506]. The incidence of oliguria was less among sPDA infants of the acetaminophen group [1 (2.3%)] than observed among the sPDA infants of the ibuprofen group [6 (14.0%)]; however, this difference was not statistically significant (P=0.108). Additionally, the incidences of fecal occult blood positive rate, intraventricular hemorrhage, neonatal necrotizing enterocolitis and bronchopulmonary dysplasia were distributed similarly in the ibuprofen and acetaminophen groups (P>0.05). The levels of platelet, serum creatinine and alanine transaminase showed no significant changes between the ibuprofen and acetaminophen groups (P>0.05). Following treatment with ibuprofen or acetaminophen, the extent of decrease of plasma and urinary PGE2 was significantly higher among sPDA infants with oliguria (135.0±38.0 ng/l) than that observed in sPDA infants without oliguria (52.5±37.0 ng/l) (P=0.01). The study also found a significant correlation between plasma and urinary PGE2 levels (r=0.648, P=0.01) and the coefficient of variation of urinary PGE2 (0.427) was less than that of plasma PGE2 (0.539). The clinical efficacy of oral ibuprofen and acetaminophen in the treatment of preterm infants with sPDA was similar with low adverse events, whereas acetaminophen-induced PGE2 levels were less than the levels observed in the ibuprofen-treated group. The incidence of oliguria was also lower in the acetaminophen group compared to the ibuprofen group. In addition, monitoring urinary PGE2 levels was more suitable because of its non-invasiveness in the clinical setting than monitoring of plasma PGE2 in preterm infants with sPDA.
Keywords: acetaminophen; ibuprofen; infant; patent ductus arteriosus; preterm; prostaglandin E2.
Figures
References
-
- Clyman RI. Patent ductus arteriosus in the preterm infant. In: Gleason CA, Devaskar S, editors. Avery's Diseases of the Newborn. 9th. Saunders; Philadelphia, PA: 2012. pp. 751–761. - DOI
-
- Gao X, Hei M, Yang B, Zhu H, Zhang QG, Lei HG, Ren Y. The changes of plasma prostaglandins E2 at pre- and post-treatment in preterm infants with patent ductus arteriosus. Chin J Heart Heart Rhythm. 2015;3:102–108.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous