Lentivirus-mediated Knockdown of HDAC1 Uncovers Its Role in Esophageal Cancer Metastasis and Chemosensitivity
- PMID: 27698906
- PMCID: PMC5039390
- DOI: 10.7150/jca.15086
Lentivirus-mediated Knockdown of HDAC1 Uncovers Its Role in Esophageal Cancer Metastasis and Chemosensitivity
Abstract
Histone deacetylationase 1 (HDAC1) is ubiquitously expressed in various cell lines and tissues and play an important role of regulation gene expression. Overexpression of HDAC1 has been observed in various types of cancers, which indicated that it might be a target for cancer therapy. To test HDAC1 inhibition for cancer treatment, the gene expression of HDAC1 was knockdown mediated by a lentivirus system. Our data showed the gene expression of HDAC1 could be efficiently knockdown by RNAi mediated by lentivirus in esophageal carcinoma EC109 cells. Knockdown of HDAC1 led to significant decrease of cell growth and altered cell cycle distribution. The result of transwell assay showed that the numbers of cells travelled through the micropore membrane was significantly decreased as HDAC1 expression was knockdown. Moreover, HDAC1 knockdown inhibited the migration of EC109 cells as determining by scratch test. Additionally, enhancement of cisplatin-stimulated apoptosis was detected by HDAC1 knockdown. Our data suggested inhibition of HDAC1 expression by lentivirus mediated shRNA might be further applied for esophageal cancer chemotherapy.
Keywords: EC109 cells; chemosensitivity; histone deacetylationase; lentivirus.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
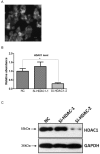

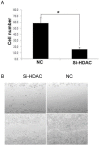

References
-
- Wagner T, Brand P, Heinzel T, Krämer OH. Histone deacetylase 2 controls p53 and is a critical factor in tumorigenesis. Biochim Biophys Acta. 2014;1846(2):524–38. - PubMed
-
- Højfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nat Rev Drug Discov. 2013;12(12):917–30. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

